Hereditary angioedema (HAE) in children and adolescents: New treatment options
- PMID: 39502954
- PMCID: PMC11536504
- DOI: 10.5414/ALX02532E
Hereditary angioedema (HAE) in children and adolescents: New treatment options
Abstract
Modern management of hereditary angioedema (HAE) due to reduced C1 inhibitor (C1-INH) function or concentration (HAE-C1-INH) focuses on individualized therapeutic strategies to address the specific needs of children and adolescents as well as the severity of the disease. Psychosocial factors such as the burden of disease and therapy on quality of life and participation play an important role. New medications have already significantly improved the prognosis and health related quality of life in HAE patients, but not all of these therapies have yet been approved for children. Further treatment options that inhibit bradykinin effects are currently being investigated. They target factor XIIa, prekallikrein, plasma kallikrein, or the bradykinin B2 receptor. Modern research focuses on oral options or long-acting parenteral therapy approaches to further optimize care and, in particular, the needs of children. There are also initial developments in the field of gene therapy, which could represent a causal treatment option for HAE in the future. This article focuses on the presentation and treatment of HAE type I (reduced C1-INH concentration) and HAE type II (impaired C1-INH function) in children and adolescents. Acquired AE and HAE with normal C1-INH are rare in the pediatric age group and are not discussed in detail here.
Keywords: bradykinin B2 receptor (BK2R) inhibition; children and adolescents; factor XIIa inhibition; gene editing; hereditary angioedema (HAE); long-term prophylaxis; on-demand treatment; plasma kallikrein and prekallikrein inhibition; quality of life.
© Dustri-Verlag Dr. K. Feistle.
Conflict of interest statement
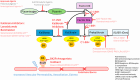
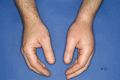
MF: personal: no conflicts of interest; institutional: IDCL participates in NIS PIQHAR (Prophylaxis Impact on Quality of life Impairment of HAE Patients with Lower Annual Base Attack Rates) BW: Lecture fees (mostly paid) from Ärztekammer Niedersachsen, ALK-Abelló, Bencard, Cogitando, BVDD, CSL Behring, DDG, DGA, DGAKI, DGP, fg-hno-aerzte, FOMF, NDG, NEDH e.V., Novartis, Roche Posay, Streamed Up, Takeda, ThermofisherScientific sowie Advisory Board (honoriert) von Biocryst, Biomarin, CSL Behring, Kalvista, Novartis, Sanofi-Aventis, Takeda. Figure 1.Pathogenesis of hereditary angioedema and therapeutic approaches, considering historical* and investigational** agents. Adapted with permission from Volker Wahn from [3] based on [5] and [6].Figure 2.HAE swelling of the left hand. Figure 3.HAE swelling of the lip.Figure 4.Erythema marginatum on the forearm preceeding an HAE swelling. Table 1.Diagnosing hereditary angioedema. Clinical symptoms/medical historyRecurrent peripheral swelling of the skin without urticaria/itching and no response to corticosteroids, epinephrine, or antihistaminesAbdominal pain attacksPossible laryngeal swellingFamily historyPossibly positive family history for HAELaboratory findingsReduced C4 and reduced C1-INH activity, with or without reduced C1-INH concentration in plasmaC1-INH activityC1-INH concentrationC4 concentrationHAE type I↓↓↓HAE type II↓N/↑↓HAE = hereditary angioedema; C1-INH = C1 inhibitor; N = normal. Table 2.On-demand treatment of acute hereditary angioedema attacks. Active ingredient/mode of action (approved in Germany)Way of administrationDosage for children by age or BWDosage for adolescents/adultsSpecial featurespdC1-INH (500/1,500) Berinert Plasma-derived C1-INHIV≥ 0 years: 20 IU/kg BW20 IU/kg BWPlasma product Reconstitution Home therapy/self-application Batch documentationpdC1-INH (500) Cinryze Plasma-derived C1-INHIV2 – 11 years (10 – 25 kg): 500 IU 2 – 11 years (> 25 kg): 1,000 IU≥ 12 years (> 25 kg): 1,000 IUPlasma product Reconstitution Home therapy/self-application Batch documentationrhC1-INH (2100) Conestat αRecombinant analogue of the human C1-INHIV≥ 2 years (< 84 kg): 50 IU/kg BW< 84kg: 50 IU/kg ≥ 84kg: 4,200 IUContains traces of rabbit protein Reconstitution Home therapy/self-applicationIcatibant Selective, competitive bradykinin type 2 receptor antagonistSC2 – 17 years • 12 – 25 kg: 10 mg (1 mL) • 26 – 40 kg: 15 mg (1.5 mL) • 41 – 50 kg: 20 mg (2 mL) • 51 – 65 kg: 25 mg (2.5 mL) • > 65 kg: 30 mg (3 mL)≥ 18 years (> 65 kg): 30 mg (3 mL)Prefilled syringe Home therapy/self-applicationC1-INH = C1 inhibitor; IV = intravenously; SC = subcutaneously; BW = body weight. According to German product information. Of note (according to [3]): – Every attack in the head and neck area should be treated at any age! – Initiate therapy as early as possible! On-demand medication should always be readily available! – Treatment of children younger than 6 years of age should also include abdominal and peripheral attacks. – Consider treatment of attacks (outside the head and neck area, depending on severity) in children older than 6 years of age. Table 3.Short-term prophylaxis prior to invasive medical procedures. Active ingredient/mode of action (approved in Germany)Way of administrationDosage for children by age or BWDosage for adolescents/adultsSpecial featurespdC1-INH (500/1,500) Berinert Plasma-derived C1-INHIV0 – < 18 years: 15 – 30 IU/kg BW< 18 years: 15 – 30 IU/kg bw ≥ 18 years: 1,000 IUPlasma product Reconstitution Home therapy/self-application Within 6 hours before the procedurepdC1-INH (500) Cinryze Plasma-derived C1-INHIV2 – 11 years (10 – 25 kg): 500 IU 2 – 11 years (> 25 kg): 1,000 IU≥ 12 years (> 25 kg): 1,000 IUPlasma product Reconstitution Home therapy/self-application Within 24 hours prior to the procedureC1-INH = C1 inhibitor; IV = intravenously; BW = body weight. According to German product information. Of note (according to [3]): – Administration prior to all invasive medical procedures in the head/neck area, and as close as possible to the procedure! – If short-term prophylaxis is not used, on-demand therapy with C1-INH concentrate should be readily available. Table 4.Long-term prophylaxis for the prevention of hereditary angioedema attacks. Active ingredient/mode of action (Approved in Germany)Way of administrationDosage for children by age or BWDosage for adolescents/adultsSpecial featurespdC1-INH (2000/3000) Berinert Plasma-derived C1-INHSC/≥ 12 years: 60 IU/kg BW 2×/weekPlasma product Reconstitution Home therapy/self-application Batch documentationpdC1-INH (500) Cinryze Plasma-derived C1-INHIV6 – 11 years: 500 IU every 3 – 4 days≥ 12 years: 1,000 IU every 3 – 4 daysPlasma product Reconstitution Home therapy/self-application Batch documentationLanadelumab (Recombinant human monoclonal antibody) Plasma kallikrein inhibitorSC2 – 11 years • 10 – ≤ 20 kg: 150 mg every 4 weeks • 20 – ≤ 40 kg: 150 mg every 2 weeks • ≥ 40 kg: 300 mg every 2 weeks≥ 12 years: 300 mg every 2 weeksPrefilled syringe Home therapy/self-application Dose adjustment possible Batch documentationBerotralstat (Small molecule) Synthetic oral plasma kallikrein inhibitorPO/≥ 12 years (≥ 40 kg): 150 mg 1 × dailyHard capsule Home therapy/self-application Consumption with a mealC1-INH = C1 inhibitor; IV = intravenously; SC = subcutaneously; PO = orally; BW = body weight. According to German product information. Of note (according to [1, 3]): Consideration of the attack frequency (generally > 2 attacks per month), individual disease burden, as well as limitations in health-related quality of life and participation due to HAE, when deciding on long-term prophylaxis.
Figures


References
-
- Maurer M Magerl M Betschel S Aberer W Ansotegui IJ Aygören-Pürsün E Banerji A Bara NA Boccon-Gibod I Bork K Bouillet L Boysen HB Brodszki N Busse PJ Bygum A Caballero T Cancian M Castaldo A Cohn DM Csuka D The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy. 2022; 77: 1961–1990. - PubMed
-
- Bork K Aygören-Pürsün E Bas M Biedermann T Greve J Hartmann K Magerl M Martinez-Saguer I Maurer M Ott H Schauf L Staubach P Wedi B Guideline: Hereditary angioedema due to C1 inhibitor deficiency. Allergo J Int. 2019; 28: 16–29.
-
- Wahn V Aberer W Aygören-Pürsün E Bork K Eberl W Faßhauer M Krüger R Magerl M Martinez-Saguer I Späth P Staubach-Renz P Weber-Chrysochoou C Hereditary angioedema in children and adolescents – A consensus update on therapeutic strategies for German-speaking countries. Pediatr Allergy Immunol. 2020; 31: 974–989. - PubMed
-
- Busse PJ Christiansen SC Hereditary Angioedema. N Engl J Med. 2020; 382: 1136–1148. - PubMed
-
- Busse P Kaplan A Specific Targeting of Plasma Kallikrein for Treatment of Hereditary Angioedema: A Revolutionary Decade. J Allergy Clin Immunol Pract. 2022; 10: 716–722. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
