Risk of Lymphedema After Sentinel Node Biopsy in Patients With Breast Cancer
- PMID: 39503367
- PMCID: PMC11543278
- DOI: 10.4048/jbc.2024.0180
Risk of Lymphedema After Sentinel Node Biopsy in Patients With Breast Cancer
Abstract
Purpose: Although numerous studies have identified potential risk factors for ipsilateral lymphedema development in patients with breast cancer following axillary node dissection, the risk factors for lymphedema in patients undergoing sentinel node biopsy without axillary dissection remain unclear. In this study, we aimed to determine the real-world incidence and risk factors for lymphedema in such patients.
Methods: We conducted a single-center, retrospective review of medical records of patients with breast cancer who underwent sentinel node biopsy alone. The development cohort (5,051 patients, January 2017-December 2020) was analyzed to identify predictors of lymphedema, and a predictive model was subsequently created. A validation cohort (1,627 patients, January 2014-December 2016) was used to validate the model.
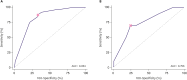
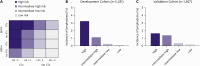
Results: In the development cohort, 49 patients (0.9%) developed lymphedema over a median follow-up of 56 months, with most cases occurring within the first three years post-operation. Multivariate analysis revealed that a body mass index (BMI) of 30 kg/m² or above, radiation therapy (RTx), chemotherapy, and more than three harvested lymph nodes significantly predicted lymphedema. The predictive model showed an area under the curve of 0.824 for systemic chemotherapy, with the number of harvested lymph nodes being the most significant factor. Patients were stratified into four risk groups, showing lymphedema incidences of 3.3% in the highest-risk group and 0.1% in the lowest-risk group. In the validation cohort, the incidences were 1.7% and 0.2% for the highest and lowest risk groups, respectively.
Conclusion: The lymphedema prediction model identifies RTx, chemotherapy, BMI ≥ 30 kg/m², and more than three harvested lymph nodes as significant risk factors. Although the overall incidence is low, the risk is notably influenced by the extent of lymph node removal and systemic therapies. The model's high negative predictive value supports its application in designing tailored lymphedema surveillance programs for early intervention.
Keywords: Lymphedema; Predictive Value of Tests; Sentinel Lymph Node Biopsy.
© 2024 Korean Breast Cancer Society.
Conflict of interest statement
Han-Byoel Lee and Wonshik Han are members of the board of directors and have stock and ownership interests at DCGen Co., Ltd. Other authors declare no conflicts of interest.
Figures
Comment in
-
Letter to the Editor: "Risk of Lymphedema After Sentinel Node Biopsy in Patients With Breast Cancer".J Breast Cancer. 2025 Feb;28(1):46-47. doi: 10.4048/jbc.2024.0286. J Breast Cancer. 2025. PMID: 40047091 Free PMC article. No abstract available.
Similar articles
-
The incidence and risk factors for occurrence of arm lymphedema after treatment of breast cancer.Chirurgia (Bucur). 2015 Jan-Feb;110(1):33-7. Chirurgia (Bucur). 2015. PMID: 25800313
-
Risk factors for breast cancer-related lymphedema: correlation with docetaxel administration.Breast Cancer. 2020 Sep;27(5):929-937. doi: 10.1007/s12282-020-01088-x. Epub 2020 Apr 8. Breast Cancer. 2020. PMID: 32270417
-
Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients.Breast J. 2010 Jan-Feb;16(1):48-54. doi: 10.1111/j.1524-4741.2009.00855.x. Epub 2009 Nov 2. Breast J. 2010. PMID: 19889169
-
The changing role of axillary treatment in breast cancer: Who will remain at risk for developing arm morbidity in the future?Breast. 2015 Oct;24(5):543-7. doi: 10.1016/j.breast.2015.04.008. Epub 2015 Jun 6. Breast. 2015. PMID: 26051795 Review.
-
Incorporating Lymphovenous Anastomosis in Clinically Node-Positive Women Receiving Neoadjuvant Chemotherapy: A Shared Decision-Making Model and Nuanced Approached to the Axilla.Curr Oncol. 2023 Apr 3;30(4):4041-4051. doi: 10.3390/curroncol30040306. Curr Oncol. 2023. PMID: 37185419 Free PMC article. Review.
Cited by
-
Letter to the Editor: "Risk of Lymphedema After Sentinel Node Biopsy in Patients With Breast Cancer".J Breast Cancer. 2025 Feb;28(1):46-47. doi: 10.4048/jbc.2024.0286. J Breast Cancer. 2025. PMID: 40047091 Free PMC article. No abstract available.
-
Reply to "Letter to the Editor: Risk of Lymphedema After Sentinel Node Biopsy in Patients With Breast Cancer".J Breast Cancer. 2025 Feb;28(1):48-49. doi: 10.4048/jbc.2024.0299. J Breast Cancer. 2025. PMID: 40047090 Free PMC article. No abstract available.
-
The Role of Axillary Lymph Node Dissection in Breast Cancer Patients With Residual Nodal Disease After Receiving Neoadjuvant Chemotherapy.Breast Cancer (Auckl). 2025 Aug 10;19:11782234251352996. doi: 10.1177/11782234251352996. eCollection 2025. Breast Cancer (Auckl). 2025. PMID: 40792125 Free PMC article.
References
-
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. - PubMed
-
- Kell MR, Burke JP, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta-analysis. Breast Cancer Res Treat. 2010;120:441–447. - PubMed
-
- Del Bianco P, Zavagno G, Burelli P, Scalco G, Barutta L, Carraro P, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella-GIVOM Italian randomised clinical trial. Eur J Surg Oncol. 2008;34:508–513. - PubMed
-
- Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment--a nationwide study of prevalence and associated factors. Breast. 2010;19:506–515. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

