DNAH3 deficiency causes flagellar inner dynein arm loss and male infertility in humans and mice
- PMID: 39503742
- PMCID: PMC11540302
- DOI: 10.7554/eLife.96755
DNAH3 deficiency causes flagellar inner dynein arm loss and male infertility in humans and mice
Abstract
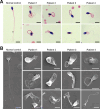
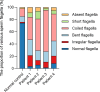
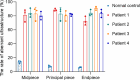
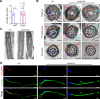
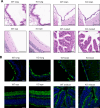
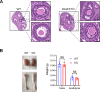
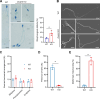
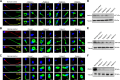
Axonemal protein complexes, including the outer and inner dynein arms (ODA/IDA), are highly ordered structures of the sperm flagella that drive sperm motility. Deficiencies in several axonemal proteins have been associated with male infertility, which is characterized by asthenozoospermia or asthenoteratozoospermia. Dynein axonemal heavy chain 3 (DNAH3) resides in the IDA and is highly expressed in the testis. However, the relationship between DNAH3 and male infertility is still unclear. Herein, we identified biallelic variants of DNAH3 in four unrelated Han Chinese infertile men with asthenoteratozoospermia through whole-exome sequencing (WES). These variants contributed to deficient DNAH3 expression in the patients' sperm flagella. Importantly, the patients represented the anomalous sperm flagellar morphology, and the flagellar ultrastructure was severely disrupted. Intriguingly, Dnah3 knockout (KO) male mice were also infertile, especially showing the severe reduction in sperm movement with the abnormal IDA and mitochondrion structure. Mechanically, nonfunctional DNAH3 expression resulted in decreased expression of IDA-associated proteins in the spermatozoa flagella of patients and KO mice, including DNAH1, DNAH6, and DNALI1, the deletion of which has been involved in disruption of sperm motility. Moreover, the infertility of patients with DNAH3 variants and Dnah3 KO mice could be rescued by intracytoplasmic sperm injection (ICSI) treatment. Our findings indicated that DNAH3 is a novel pathogenic gene for asthenoteratozoospermia and may further contribute to the diagnosis, genetic counseling, and prognosis of male infertility.
Keywords: DNAH3; asthenoteratozoospermia; genetics; genomics; human; inner dynein arm; male infertility; mouse.
© 2024, Wang, Shen, Yang et al.
Conflict of interest statement
XW, GS, YY, CJ, TR, XY, LZ, YZ, YO, XZ, SL, XT, TL, YS No competing interests declared
Figures





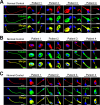
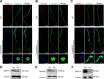


Update of
- doi: 10.1101/2024.02.19.580977
- doi: 10.7554/eLife.96755.1
- doi: 10.7554/eLife.96755.2
- doi: 10.7554/eLife.96755.3
References
-
- Aprea I, Raidt J, Höben IM, Loges NT, Nöthe-Menchen T, Pennekamp P, Olbrich H, Kaiser T, Biebach L, Tüttelmann F, Horvath J, Schubert M, Krallmann C, Kliesch S, Omran H. Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLOS Genetics. 2021;17:e1009306. doi: 10.1371/journal.pgen.1009306. - DOI - PMC - PubMed
-
- Ben Khelifa M, Coutton C, Zouari R, Karaouzène T, Rendu J, Bidart M, Yassine S, Pierre V, Delaroche J, Hennebicq S, Grunwald D, Escalier D, Pernet-Gallay K, Jouk P-S, Thierry-Mieg N, Touré A, Arnoult C, Ray PF. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. American Journal of Human Genetics. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

