Cytosolic N6AMT1- dependent translation supports mitochondrial RNA processing
- PMID: 39503847
- PMCID: PMC11588129
- DOI: 10.1073/pnas.2414187121
Cytosolic N6AMT1- dependent translation supports mitochondrial RNA processing
Abstract
Mitochondrial biogenesis relies on both the nuclear and mitochondrial genomes, and imbalance in their expression can lead to inborn errors of metabolism, inflammation, and aging. Here, we investigate N6AMT1, a nucleo-cytosolic methyltransferase that exhibits genetic codependency with mitochondria. We determine transcriptional and translational profiles of N6AMT1 and report that it is required for the cytosolic translation of TRMT10C (MRPP1) and PRORP (MRPP3), two subunits of the mitochondrial RNAse P enzyme. In the absence of N6AMT1, or when its catalytic activity is abolished, RNA processing within mitochondria is impaired, leading to the accumulation of unprocessed and double-stranded RNA, thus preventing mitochondrial protein synthesis and oxidative phosphorylation, and leading to an immune response. Our work sheds light on the function of N6AMT1 in protein synthesis and highlights a cytosolic program required for proper mitochondrial biogenesis.
Keywords: OXPHOS; RNA processing; mitochondria; mitochondrial RNA granules; translation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

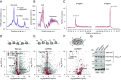
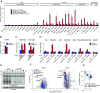

References
-
- Wu Z., et al. , Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999). - PubMed
-
- Virbasius J. V., Scarpulla R. C., Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. U.S.A. 91, 1309–1313 (1994). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

