Safety of Simultaneous vs Sequential mRNA COVID-19 and Inactivated Influenza Vaccines: A Randomized Clinical Trial
- PMID: 39504023
- PMCID: PMC11541642
- DOI: 10.1001/jamanetworkopen.2024.43166
Safety of Simultaneous vs Sequential mRNA COVID-19 and Inactivated Influenza Vaccines: A Randomized Clinical Trial
Abstract
Importance: Limited randomized clinical trial data exist on the safety of simultaneous administration of COVID-19 and influenza vaccines.
Objective: To compare the reactogenicity, safety, and changes in health-related quality of life (HRQOL) after simultaneous vs sequential receipt of messenger RNA (mRNA) COVID-19 vaccine and quadrivalent inactivated influenza vaccine (IIV4).
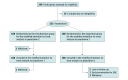
Design, setting, and participants: This randomized, placebo-controlled clinical trial was conducted between October 8, 2021, and June 14, 2023, at 3 US sites. Participants were nonpregnant persons aged 5 years or older with the intention of receiving both influenza and mRNA COVID-19 vaccines.
Interventions: Intramuscular administration in opposite arms of either IIV4 or saline placebo simultaneously with mRNA COVID-19 vaccine at visit 1. Those who received placebo at visit 1 received IIV4 and those who received IIV4 at visit 1 received placebo 1 to 2 weeks later at visit 2.
Main outcomes and measures: The primary composite reactogenicity outcome was the proportion of participants with fever, chills, myalgia, and/or arthralgia of moderate or greater severity within 7 days after vaccination visits 1 and/or 2, using a 10% noninferiority margin. Secondary outcomes were solicited reactogenicity events and unsolicited adverse events (AEs) for 7 days after each visit separately and HRQOL after visit 1, assessed by the EuroQol 5-Dimension 5-Level (EQ-5D-5L) Index. Serious AEs (SAEs) and AEs of special interest (AESIs) were assessed for 121 days. Outcomes were compared between groups.
Results: A total of 335 persons (mean [SD] age, 33.4 [15.1] years) were randomized (169 to the simultaneous group and 166 to the sequential group); 211 (63.0%) were female, and 255 (76.1%) received bivalent BNT162b2 mRNA COVID-19 vaccine. The proportion with the primary composite reactogenicity outcome in the simultaneous group (25.6% [n = 43]) was noninferior to the proportion in the sequential group (31.3% [n = 52]) (site-adjusted difference, -5.6 percentage points [pp]; 95% CI, -15.2 to 4.0 pp). Respective proportions in each group were similar after each visit separately (visit 1, 40 [23.8%] vs 47 [28.3%]; visit 2, 5 [3.0%] vs 9 [5.4%]). No significant group differences in participants with AEs (21 [12.4%] vs 16 [9.6%]), SAEs (1 [0.6%] vs 1 [0.6%]), and AESIs (19 [11.2%] vs 9 [5.4%]) were observed in the simultaneous vs sequential groups, respectively. Among participants with severe reactogenicity, the mean (SD) EQ-5D-5L Index score decreased from 0.92 (0.08) to 0.92 (0.09) prevaccination to 0.81 (0.09) to 0.82 (0.12) postvaccination.
Conclusions and relevance: In this randomized clinical trial assessing simultaneous vs sequential administration of mRNA COVID-19 and IIV4 vaccines, reactogenicity was comparable in both groups. These findings support the option of simultaneous administration of these vaccines.
Trial registration: ClinicalTrials.gov Identifier: NCT05028361.
Conflict of interest statement
Figures


References
-
- Flu burden prevented from vaccination 2021-2022 flu season. Centers for Disease Control and Prevention. Accessed September 26, 2024. https://www.cdc.gov/flu-burden/php/data-vis-vac/2021-2022-prevented.html
-
- Johnson AG, Amin AB, Ali AR, et al. ; MSHI . COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132-138. doi: 10.15585/mmwr.mm7104e2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

