Decision Support Intervention and Anticoagulation for Emergency Department Atrial Fibrillation: The O'CAFÉ Stepped-Wedge Cluster Randomized Clinical Trial
- PMID: 39504024
- PMCID: PMC11541643
- DOI: 10.1001/jamanetworkopen.2024.43097
Decision Support Intervention and Anticoagulation for Emergency Department Atrial Fibrillation: The O'CAFÉ Stepped-Wedge Cluster Randomized Clinical Trial
Abstract
Importance: Oral anticoagulation for adults with atrial fibrillation or atrial flutter (AFF) who are at elevated stroke risk reduces the incidence of ischemic stroke but remains underused. Efforts to increase anticoagulation initiation on emergency department (ED) discharge have yielded conflicting results.
Objective: To evaluate the effectiveness of a multipronged intervention supporting anticoagulation initiation for eligible adult ED patients.
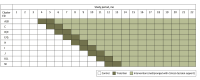
Design, setting, and participants: The Clinical Decision Support to Optimize Care of Patients With Atrial Fibrillation or Flutter in the Emergency Department (O'CAFÉ) pragmatic, stepped-wedge cluster randomized clinical trial was conducted from July 1, 2021, through April 30, 2023, at 13 community medical centers (in 9 clusters) of an integrated health system in Northern California. The study included adult ED patients with primary AFF eligible for anticoagulation initiation when discharged home. Clusters were randomly assigned to staggered dates for 1-way crossover from the control phase (usual care) to the intervention phase.
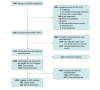
Intervention: Physician education, facility-specific audit and feedback, and access to decision support, which identified eligible patients and recommended shared decision-making, anticoagulation initiation (if suitable), and timely follow-up.
Main outcomes and measures: The main outcome was a composite of anticoagulation on discharge or within 30 days. A primary intention-to-treat analysis (decision support access regardless of use) and a secondary per-protocol analysis (decision support use) were performed. Multivariable analyses adjusted for intervention and exposure months with random effects, accounting for clustering by facility and patient.
Results: A total of 3388 eligible patients with atrial fibrillation were discharged home: 2185 (64.5%) were receiving pre-ED arrival anticoagulation and 1203 (35.5%) were eligible for anticoagulation. Among the 1203 patients with an initiation-eligible encounter, the median age was 74.0 (IQR, 68.0-82.0) years and approximately half (618 [51.4%]) were men. Among the 387 patients with an initiation-eligible control encounter, 244 (63.0%) received anticoagulation (190 [49.0%] at discharge and 54 [14.0%] within 30 days). Among the 816 patients with an initiation-eligible intervention encounter, 558 (68.4%) received anticoagulation (428 [52.5%] on discharge and 130 [15.9%] within 30 days). There was no statistically significant change in initiation of anticoagulation associated with the intervention (adjusted odds ratio, 1.33 [95% CI, 0.75-2.35]; P = .13). Decision support was used for 217 eligible case patients (26.6%) (per protocol) and was associated with a statistically significant change in anticoagulation initiation when compared with 599 patients for whom decision support was not used (164 [75.6%] vs 394 [65.8%]; P = .008).
Conclusions and relevance: In this trial, a multipronged intervention to facilitate thromboprophylaxis among eligible ED patients with AFF did not significantly increase anticoagulation initiation. Opportunities exist to further improve stroke prevention among ED patients with primary AFF.
Trial registration: ClinicalTrials.gov Identifier: NCT05009225.
Conflict of interest statement
Figures


References
-
- Joglar JA, Chung MK, Armbruster AL, et al. ; Writing Committee Members . 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2024;83(1):109-279. doi:10.1016/j.jacc.2023.08.017 - DOI - PMC - PubMed
-
- January CT, Wann LS, Alpert JS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. doi:10.1016/j.jacc.2014.03.022 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

