PINK1/Parkin-Mediated Mitophagy Ameliorates Mitochondrial Dysfunction in Lacrimal Gland Acinar Cells During Aging
- PMID: 39504053
- PMCID: PMC11549928
- DOI: 10.1167/iovs.65.13.12
PINK1/Parkin-Mediated Mitophagy Ameliorates Mitochondrial Dysfunction in Lacrimal Gland Acinar Cells During Aging
Abstract
Purpose: Aging alters the function of the lacrimal gland and disrupts the balance of the microenvironment on the ocular surface, eventually leading to aqueous-tear-deficient dry eye. Mitophagy has been reported to play an important role in aging, but the underlying mechanism remains unclear.
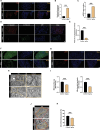
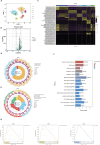
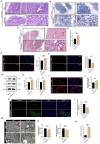
Methods: The young (6 weeks) and middle-aged (12 months) male C57BL/6J mice were used in this study, and mitophagy agonist rapamycin and inhibitor Mdivi-1 were used in in vivo experiments. Hematoxylin and eosin, Masson, Oil Red O, and reactive oxygen species (ROS) staining were used to detect histological changes and lipids in lacrimal gland. Changes in the expression of proteins were identified by Western blotting of lacrimal gland lysates. Transmission electron microscopy and immunofluorescence staining were used to assess mitophagy. The single-cell RNA sequencing (scRNA-seq) and bioinformatics analyses were used to detect transcription signature changes during aging.
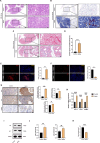
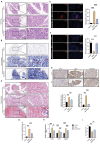
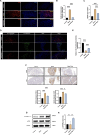
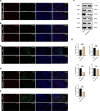
Results: In this study, we discovered that aging increased oxidative stress, which increased apoptosis, and generated ROS in acinar epithelial cells. Furthermore, activation of PINK1/Parkin-mediated mitophagy by rapamycin reduced lacrimal gland ROS concentrations and prevented aging-induced apoptosis of acinar cells, thereby causing histological alterations, microstructural degradation, and increasing tear secretion associated with ROS accumulation. By contrast, Mdivi-1 aggregates mitochondrial function and thereafter leads to lacrimal gland function impairment by inhibiting mitochondrial fission and giving rise to mitophagy.
Conclusions: Overall, our findings suggested that aging could impair mitochondrial function of acinar cells, and age-related alterations may be treated with therapeutic approaches that enhance mitophagy while maintaining mitochondrial function.
Conflict of interest statement
Disclosure:
Figures



References
-
- Dzau VJ, Inouye SK, Rowe JW, Finkelman E, Yamada T.. Enabling healthful aging for all - the National Academy of Medicine Grand Challenge in Healthy Longevity. N Engl J Med. 2019; 381: 1699–1701. - PubMed
-
- Gipson IK. Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 2013; 54: Orsf48–Orsf53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

