Psoriasis Risk With Immune Checkpoint Inhibitors
- PMID: 39504056
- PMCID: PMC11541743
- DOI: 10.1001/jamadermatol.2024.4129
Psoriasis Risk With Immune Checkpoint Inhibitors
Abstract
Importance: Immune checkpoint inhibitors (ICIs) are recognized as revolutionary cancer therapies but have raised concerns about immune-related adverse events, including the development of autoimmune diseases.
Objective: To evaluate the psoriasis risk associated with the use of ICIs in patients with cancer.
Design, setting, and participants: This nationwide cohort study with a target trial emulation design used data from the Taiwan National Health Insurance database and the Taiwan Cancer Registry. The participants included were patients who received antineoplastic medications for cancer at stages III and IV between January 1, 2019, and June 30, 2021. Data were analyzed from May 2023 to July 2024.
Exposures: Patients treated with ICIs were classified as ICI users, while those who received chemotherapy or targeted therapies were categorized as non-ICI users.
Main outcome and measures: The primary outcome was the incidence of psoriasis during the follow-up period. Stabilized inverse probability of treatment weighting (IPTW) was used to mitigate potential confounders. Cox and Fine-Gray hazard models were used to calculate hazard ratios (HRs) for psoriasis risk between groups.
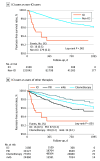
Results: Of 135 230 patients who received antineoplastic medications (mean [SD] age, 62.94 [13.01] years; 45.1% female), 3188 patients were eligible for the ICI user group, while 132 042 patients were eligible for the non-ICI user group. ICI users experienced a higher incidence of psoriasis at 5.76 cases per 1000 person-years, compared to 1.44 cases in the non-ICI group. After adjusting for demographics and comorbidities, ICI users were found to have a 2-fold increase in the risk of developing psoriasis (IPTW-adjusted HR, 3.31; IPTW-adjusted subdistribution HR, 2.43). Both as-started design and on-treatment design showed consistent findings, and the results were consistent and robust across all follow-up intervals and all sensitivity analyses.
Conclusions and relevance: In this cohort study, patients with cancer treated with ICIs faced an increased risk of psoriasis. Medical professionals should be aware of the potential adverse effects of immunotherapy to ensure optimal cancer care.
Conflict of interest statement
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

