Insights From the Development of a Dynamic Consent Platform for the Australians Together Health Initiative (ATHENA) Program: Interview and Survey Study
- PMID: 39504120
- PMCID: PMC11579620
- DOI: 10.2196/57165
Insights From the Development of a Dynamic Consent Platform for the Australians Together Health Initiative (ATHENA) Program: Interview and Survey Study
Abstract
Background: Dynamic consent has the potential to address many of the issues facing traditional paper-based or electronic consent, including enrolling informed and engaged participants in the decision-making process. The Australians Together Health Initiative (ATHENA) program aims to connect participants across Queensland, Australia, with new research opportunities. At its core is dynamic consent, an interactive and participant-centric digital platform that enables users to view ongoing research activities, update consent preferences, and have ongoing engagement with researchers.
Objective: This study aimed to describe the development of the ATHENA dynamic consent platform within the framework of the ATHENA program, including how the platform was designed, its utilization by participants, and the insights gained.
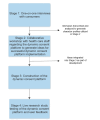
Methods: One-on-one interviews were undertaken with consumers, followed by a workshop with health care staff to gain insights into the dynamic consent concept. Five problem statements were developed, and solutions were posed, from which a dynamic consent platform was constructed, tested, and used for implementation in a clinical trial. Potential users were randomly recruited from a pre-existing pool of 615 participants in the ATHENA program. Feedback on user platform experience was gained from a survey hosted on the platform.
Results: In the 13 consumer interviews undertaken, participants were positive about dynamic consent, valuing privacy, ease of use, and adequate communication. Motivators for registration were feedback on data usage and its broader community benefits. Problem statements were security, trust and governance, ease of use, communication, control, and need for a scalable platform. Using the newly constructed dynamic consent platform, 99 potential participants were selected, of whom 67 (68%) were successfully recontacted. Of these, 59 (88%) agreed to be sent the platform, 44 (74%) logged on (indicating use), and 22 (57%) registered for the clinical trial. Survey feedback was favorable, with an average positive rating of 78% across all questions, reflecting satisfaction with the clarity, brevity, and flexibility of the platform. Barriers to implementation included technological and health literacy.
Conclusions: This study describes the successful development and testing of a dynamic consent platform that was well-accepted, with users recognizing its advantages over traditional methods of consent regarding flexibility, ease of communication, and participant satisfaction. This information may be useful to other researchers who plan to use dynamic consent in health care research.
Keywords: clinical trials; communication; consumer engagement; decision; decision making; development; digital consent; dynamic consent; feedback; research; user platform; users.
©Eddy Xiong, Carissa Bonner, Amanda King, Zoltan Maxwell Bourne, Mark Morgan, Ximena Tolosa, Tony Stanton, Kim Greaves. Originally published in JMIR Formative Research (https://formative.jmir.org), 06.11.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Kaye J, Whitley EA, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: a patient interface for twenty-first century research networks. Eur J Hum Genet. 2015 Feb;23(2):141–6. doi: 10.1038/ejhg.2014.71. https://europepmc.org/abstract/MED/24801761 ejhg201471 - DOI - PMC - PubMed
-
- Williams H, Spencer K, Sanders C, Lund D, Whitley EA, Kaye J, Dixon WG. Dynamic consent: a possible solution to improve patient confidence and trust in how electronic patient records are used in medical research. JMIR Med Inform. 2015 Jan 13;3(1):e3. doi: 10.2196/medinform.3525. https://medinform.jmir.org/2015/1/e3/ v3i1e3 - DOI - PMC - PubMed
-
- Budin-Ljøsne I, Teare HJA, Kaye J, Beck S, Bentzen HB, Caenazzo L, Collett C, D'Abramo F, Felzmann H, Finlay T, Javaid MK, Jones E, Katić V, Simpson A, Mascalzoni D. Dynamic Consent: a potential solution to some of the challenges of modern biomedical research. BMC Med Ethics. 2017 Jan 25;18(1):4. doi: 10.1186/s12910-016-0162-9. https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-016-0162-9 10.1186/s12910-016-0162-9 - DOI - DOI - PMC - PubMed
-
- Javaid MK, Forestier-Zhang L, Watts L, Turner A, Ponte C, Teare H, Gray D, Gray N, Popert R, Hogg J, Barrett J, Pinedo-Villanueva R, Cooper C, Eastell R, Bishop N, Luqmani R, Wordsworth P, Kaye J. The RUDY study platform - a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis. 2016 Nov 08;11(1):150. doi: 10.1186/s13023-016-0528-6. https://ojrd.biomedcentral.com/articles/10.1186/s13023-016-0528-6 10.1186/s13023-016-0528-6 - DOI - DOI - PMC - PubMed
-
- Mardani A, Nakhoda M, Noruzi A, Shamsi Gooshki E. Ethical considerations in the biomedical research: analysis of national biomedical research ethics guidelines in Iran. J Med Ethics Hist Med. 2019;12:4. https://europepmc.org/abstract/MED/31346397 - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

