Protocol to study monocyte transmigration across primary human liver endothelial cells under physiological shear flow conditions in vitro
- PMID: 39504247
- PMCID: PMC11577181
- DOI: 10.1016/j.xpro.2024.103431
Protocol to study monocyte transmigration across primary human liver endothelial cells under physiological shear flow conditions in vitro
Abstract
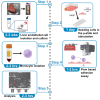
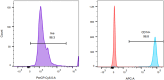
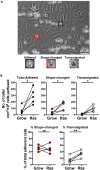
Modeling immune cell recruitment by liver endothelial cells in vitro is important to better understand the pathology of chronic inflammatory liver diseases and cancers. Here, we present a protocol for the study of monocyte transmigration across activated primary human liver endothelial cells, under physiological flow conditions. We describe primary endothelial cell isolation from human liver tissues and monocyte isolation from human blood. We then detail the shear flow-based assay and subsequent analysis of the different stages of monocyte transmigration. For complete details on the use and execution of this protocol, please refer to Wilkinson et al.1.
Keywords: Cell isolation; Cell-based Assays; Immunology.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.S. is a consultant for Faron Pharmaceuticals and Simbec-Orion. C.J.W. is a consultant for Vantage Biosciences.
Figures



References
-
- Wilkinson A.L., Hulme S., Kennedy J.I., Mann E.R., Horn P., Shepherd E.L., Yin K., Zaki M.Y.W., Hardisty G., Lu W.-Y., et al. The senescent secretome drives PLVAP expression in cultured human hepatic endothelial cells to promote monocyte transmigration. iScience. 2023;26 doi: 10.1016/j.isci.2023.107966. - DOI - PMC - PubMed
-
- Patten D.A., Wilson G.K., Bailey D., Shaw R.K., Jalkanen S., Salmi M., Rot A., Weston C.J., Adams D.H., Shetty S. Human liver sinusoidal endothelial cells promote intracellular crawling of lymphocytes during recruitment: A new step in migration. Hepatology. 2017;65:294–309. doi: 10.1002/hep.28879. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

