A Prospective Analysis of Per- and Polyfluoroalkyl Substances from Early Pregnancy to Delivery in the Atlanta African American Maternal-Child Cohort
- PMID: 39504273
- PMCID: PMC11540153
- DOI: 10.1289/EHP14334
A Prospective Analysis of Per- and Polyfluoroalkyl Substances from Early Pregnancy to Delivery in the Atlanta African American Maternal-Child Cohort
Abstract
Background: Longitudinal trends in per- and polyfluoroalkyl substances (PFAS) serum concentrations across pregnancy have not been thoroughly examined, despite evidence linking prenatal PFAS exposures with adverse birth outcomes.
Objectives: We sought to characterize longitudinal PFAS concentrations across pregnancy and to examine the maternal-fetal transfer ratio among participants in a study of risk and protective factors for adverse birth outcomes among African Americans.
Methods: In the Atlanta African American Maternal-Child cohort (2014-2020), we quantified serum concentrations of four PFAS in 376 participants and an additional eight PFAS in a subset of 301 participants during early (8-14 weeks gestation) and late pregnancy (24-30 weeks gestation). Among these, PFAS concentrations were also measured among 199 newborns with available dried blood spot (DBS) samples. We characterized the patterns, variability, and associations in PFAS concentrations at different time points across pregnancy using intraclass correlation coefficients (ICCs), maternal-newborn pairs transfer ratios, linear mixed effect models, and multivariable linear regression, adjusting for socioeconomic and prenatal predictors.
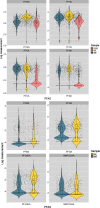
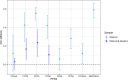
Results: Perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA) were detected in of maternal samples, with PFHxS and PFOS having the highest median concentrations. We observed high variability in PFAS concentrations across pregnancy time points (). All median PFAS concentrations increased from early to late pregnancy, except for PFOA and N-methyl perfluorooctane sulfonamido acetic acid (NMFOSAA), which decreased [paired -test for all PFAS except for PFOA and perfluorobutane sulfonic acid (PFBS)]. Prenatal serum PFAS were weakly to moderately correlated with newborn DBS PFAS ( ). The median maternal-fetal PFAS transfer ratio was lower for PFAS with longer carbon chains. After adjusting for socioeconomic and prenatal predictors, in linear mixed effect models, the adjusted mean PFAS concentrations significantly increased during pregnancy, except for PFOA. In multivariable linear regression, PFAS concentrations in early pregnancy significantly predicted the PFAS concentrations in late pregnancy and in newborns.
Discussion: We found that the concentrations of most PFAS increased during pregnancy, and the magnitude of variability differed by individual PFAS. Future studies are needed to understand the influence of within-person PFAS variability during and after pregnancy on birth outcomes. https://doi.org/10.1289/EHP14334.
Figures


References
-
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: 30470793, 10.1038/s41370-018-0094-1. - DOI - PMC - PubMed
-
- ATSDR (Agency for Toxic Substances and Disease Registry). 2022. Per- and Polyfluoroalkyl Substances (PFAS) and Your Health. What are the health effects of PFAS? https://www.atsdr.cdc.gov/pfas/health-effects/index.html [accessed 30 October 2024].
MeSH terms
Substances
Grants and funding
- R01 NR014800/NR/NINR NIH HHS/United States
- U2C ES026542/ES/NIEHS NIH HHS/United States
- R01 MD009064/MD/NIMHD NIH HHS/United States
- R01 MD009746/MD/NIMHD NIH HHS/United States
- R21 ES032117/ES/NIEHS NIH HHS/United States
- U2C ES026560/ES/NIEHS NIH HHS/United States
- R01 ES035738/ES/NIEHS NIH HHS/United States
- P30 ES019776/ES/NIEHS NIH HHS/United States
- R24 ES029490/ES/NIEHS NIH HHS/United States
- U24 ES029490/ES/NIEHS NIH HHS/United States
- T32 ES012870/ES/NIEHS NIH HHS/United States
- UH3 OD023318/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
