Urine lipoarabinomannan concentrations among HIV-negative adults with pulmonary or extrapulmonary tuberculosis disease in Vietnam
- PMID: 39504319
- PMCID: PMC11540228
- DOI: 10.1371/journal.pgph.0003891
Urine lipoarabinomannan concentrations among HIV-negative adults with pulmonary or extrapulmonary tuberculosis disease in Vietnam
Abstract
Lipoarabinomannan (LAM) is a promising target biomarker for diagnosing subclinical and clinical tuberculosis (TB). Urine LAM (uLAM) testing using rapid diagnostic tests (RDTs) has been approved for people living with HIV (PLWH), however there is limited data regarding uLAM levels in HIV-negative (HIV-ve) adults with clinical TB. We conducted a clinical study of adults presenting with clinical TB-related symptoms at the National Lung Hospital in Hanoi, Vietnam. The uLAM concentrations were measured using electrochemiluminescent (ECL) immunoassays and compared to a microbiological reference standard (MRS) using GeneXpert Ultra and TB culture testing. Estimated uLAM concentrations above plate specific calculated limit of detection (LOD) were considered uLAM positive. Additional microbiological testing was conducted for possible extrapulmonary TB (EPTB). Among 745 participants enrolled, 335 (44.9%) participants with presumptive pulmonary TB (PTB) and 6 (11.3%) participants with presumptive EPTB had confirmed TB disease. Overall, the S/A antibody pair had a sensitivity of 39% (95% Confidence Interval [CI] 0.33, 0.44) and a specificity of 97% (95% CI 0.96, 0.99) compared to the MRS. The F/A antibody pair had a sensitivity of 41% (95% CI 0.35, 0.47) and a specificity of 79% (95% CI 0.75, 0.84). S/A provided greater discriminatory ability compared to F/A for both individuals with presumptive PTB (AUROC: 0.74 vs 0.63, p<0.0001) and presumptive EPTB (0.76 vs 0.54, p = 0.045) when using the MRS. Among HIV-ve participants in an adult cohort in Vietnam, the concentrations of uLAM remained relatively low for people with clinical TB, which may present challenges for improving RDT sensitivity.
Copyright: © 2024 Hoa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
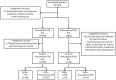
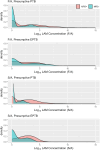

References
-
- Global Tuberculosis Report 2023. [cited 31 Jul 2024]. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa...
-
- UNITAID 2017 TUBERCULOSIS Diagnostics Technology Landscape 5th Edition, May 2017. [cited 22 Aug 2022]. https://unitaid.org/assets/2017-Unitaid-TB-Diagnostics-Technology-Landsc...
Grants and funding
LinkOut - more resources
Full Text Sources
