The transmembrane domain of the desmosomal cadherin desmoglein-1 governs lipid raft association to promote desmosome adhesive strength
- PMID: 39504468
- PMCID: PMC11656464
- DOI: 10.1091/mbc.E24-05-0200
The transmembrane domain of the desmosomal cadherin desmoglein-1 governs lipid raft association to promote desmosome adhesive strength
Abstract
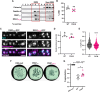
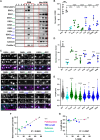
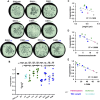
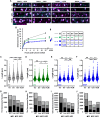
Cholesterol- and sphingolipid-enriched domains called lipid rafts are hypothesized to selectively coordinate protein complex assembly within the plasma membrane to regulate cellular functions. Desmosomes are mechanically resilient adhesive junctions that associate with lipid raft membrane domains, yet the mechanisms directing raft association of the desmosomal proteins, particularly the transmembrane desmosomal cadherins, are poorly understood. We identified the desmoglein-1 (DSG1) transmembrane domain (TMD) as a key determinant of desmoglein lipid raft association and designed a panel of DSG1TMD variants to assess the contribution of TMD physicochemical properties (length, bulkiness, and palmitoylation) to DSG1 lipid raft association. Sucrose gradient fractionations revealed that TMD length and bulkiness, but not palmitoylation, govern DSG1 lipid raft association. Further, DSG1 raft association determines plakoglobin recruitment to raft domains. Super-resolution imaging and functional assays uncovered a strong relationship between the efficiency of DSG1TMD lipid raft association and the formation of morphologically and functionally robust desmosomes. Lipid raft association regulated both desmosome assembly dynamics and DSG1 cell surface stability, indicating that DSG1 lipid raft association is required for both desmosome formation and maintenance. These studies identify the biophysical properties of desmoglein transmembrane domains as key determinants of lipid raft association and desmosome adhesive function.
Conflict of interest statement
Conflicts of interests: The authors declare no financial conflict of interest.
Figures



Update of
-
The transmembrane domain of the desmosomal cadherin desmoglein-1 governs lipid raft association to promote desmosome adhesive strength.bioRxiv [Preprint]. 2024 Apr 26:2024.04.24.590936. doi: 10.1101/2024.04.24.590936. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Dec 1;35(12):ar152. doi: 10.1091/mbc.E24-05-0200. PMID: 38712246 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

