Donor age contributes more to the rheological properties of stored red blood cells than donor sex and biological age distribution
- PMID: 39504562
- PMCID: PMC11869869
- DOI: 10.1182/bloodadvances.2024014475
Donor age contributes more to the rheological properties of stored red blood cells than donor sex and biological age distribution
Abstract
The quality of stored red cell concentrates (RCCs) has been linked to the biological age distribution of red blood cell (RBC) subpopulations. Teenage male RCCs contain higher proportions of biologically old RBCs, with poorer quality. This study sought to assess the contribution of donor sex and age on the deformability characteristics of RBC subpopulations in stored RCCs. On days 5, 14, 28, and 42 of hypothermic storage, RCCs from healthy teenage male (n = 15), senior male (n = 15), teenage female (n = 15), and senior female (n = 15) donors were biologically age profiled. The deformability of the resulting young RBCs and old RBCs (O-RBCs) was assessed using ektacytometry. Over storage, donor age was the biggest factor influencing the rheology of RBC subpopulations. Teenage male RCCs had the largest reduction in Ohyper (osmolality in the hypertonic region corresponding to 50% of the maximum RBC elongation [EImax]). The strongest correlations between Ohyper and mean corpuscular hemoglobin content (R2 > 0.5) were witnessed with O-RBCs from senior donors, and to a lesser extent with teenage males. Teen O-RBCs, particularly from males, had higher elongation indices, both under isotonic conditions and in the presence of an increasing osmotic gradient. Teen RBCs, regardless of biological age, were discovered to be more rigid (higher shear stress required to reach half the EImax). Donor variation in the age distribution of RBC subpopulations and its downstream effect on deformability serves as further evidence that factors beyond storage could potentially affect RCC quality and transfusion outcomes.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


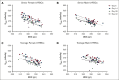
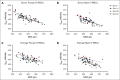
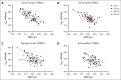
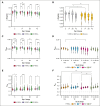
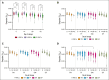
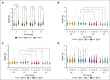
Comment in
-
How donor age affects RBC deformability in storage.Blood Adv. 2025 Feb 25;9(4):818-819. doi: 10.1182/bloodadvances.2024015257. Blood Adv. 2025. PMID: 39946162 Free PMC article.
References
-
- Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56(6):1274–1286. - PubMed
-
- Turner T, Lautner L, Hill A, Howell A, Skeate R, Acker JP. Evaluating the quality of red blood cell concentrates irradiated before or after cryopreservation. Transfusion (Paris) 2020;60(1):26–29. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

