METTL3 methylated KIF15 promotes nasopharyngeal carcinoma progression and radiation resistance by blocking ATG7-mediated autophagy through the activation of STAT3 pathway
- PMID: 39504712
- PMCID: PMC11570775
- DOI: 10.1016/j.tranon.2024.102161
METTL3 methylated KIF15 promotes nasopharyngeal carcinoma progression and radiation resistance by blocking ATG7-mediated autophagy through the activation of STAT3 pathway
Abstract
Background: Resistance to radiotherapy is a major component in the failure of nasopharyngeal carcinoma (NPC) treatment. Enhancing autophagy in nasopharyngeal carcinoma may increase its radiation sensitivity, making it critical to find autophagy-modulating targets.
Methods: The level of KIF15 was determined in NPC patients. Then, radiation-resistant NPC cells were produced to explore the mechanism in NPC. KIF15 was suppressed, and cell function and autophagy-related variables were examined in radiation-resistant NPC cells. Then the autophagy pathway was blocked, and the link between KIF15 and autophagy was confirmed. Finally, an NPC murine model was established, with tumors implanted in aberrant sites, and the relationship discovered at the cell level was confirmed in vivo. All statistical significance was determined using the student's t-test and one-way ANOVA.
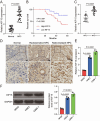
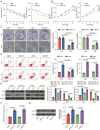
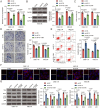
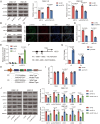
Results: Elevated amounts of KIF15 were discovered to be significantly expressed in NPC tissues and played a role in the radioresistance of NPC, a phenomenon attributed to METTL3-mediated m6A methylation. Blocking KIF15 resulted in decreased cell proliferation, increased cell death, and the activation of autophagy, ultimately making NPC more sensitive to radiation. This also resulted in decreased tumor development and increased levels of autophagy and apoptosis in vivo KIF15 interacted with STAT3, retaining it in the cytoplasm. Overexpression of STAT3 reversed the inhibitory effects of KIF15 knockdown on NPC and also reversed the influence of sh-KIF15 on autophagy activation. Inhibition of KIF15 decreased the inhibitory effect of STAT3 on ATG7, thereby upregulating autophagy activation in radio-resistant NPC cells.
Conclusion: The increased expression of KIF15 was found to be associated with the progression of NPC and play a role in the development of radioresistance in NPC. Inhibiting KIF15 was shown to impede tumor growth and improve the sensitivity of NPC to radiotherapy by triggering autophagy via the STAT3/ATG7 pathway.
Keywords: Autophagy; KIF15; NPC; Radioresistance.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Lee A.W.M., Ng W.T., Chan J.Y.W., Corry J., Makitie A., Mendenhall W.M., Rinaldo A., Rodrigo J.P., Saba N.F., Strojan P., Suarez C., Vermorken J.B., Yom S.S., Ferlito A. Management of locally recurrent nasopharyngeal carcinoma. Cancer. Treat. Rev. 2019 doi: 10.1016/j.ctrv.2019.101890. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

