Influence of cocaine use reduction on markers of immune function
- PMID: 39504756
- PMCID: PMC11620913
- DOI: 10.1016/j.jneuroim.2024.578470
Influence of cocaine use reduction on markers of immune function
Abstract
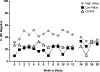
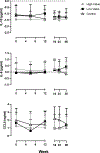
This study determined the effects of reduced cocaine use on immune function. Treatment seeking participants with Cocaine Use Disorder enrolled in a 12-week contingency management trial to reduce cocaine use. Participants were randomly assigned 1:1:1 to High Value Reinforcers (i.e., $55/negative urine sample) for cocaine abstinence (n = 41), Low Value Reinforcers (i.e., $13/negative urine sample) for cocaine abstinence (n = 33) or Non-Contingent Control (n = 33). Immune measures were collected at 6-week intervals. The High Value group had greatest use reductions, increased erythema and IL-6 and decreased IL-10 and CCL5, suggesting an activated immune response. Cocaine use reduction may promote changes in immune health.
Trial registration: ClinicalTrials.gov NCT03224546.
Keywords: Clinical trial; Cocaine; Human; Immune.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures


References
-
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
-
- Aminesmaeili M, Farokhnia M, Susukida R, Leggio L, Johnson RM, Crum RM, Mojtabai R (2024). Reduced drug use as an alternative outcome in individuals with stimulant use disorders: Findings from 13 multisite randomized clinical trials. Addiction. https://onlinelibrary.wiley.com/doi/10.1111/add.16409 - DOI - PMC - PubMed
-
- Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, Campos-Cloute R, Ruiz JJ, Romero P, Suárez J, Baixeras E, de la. Torre R, Montesinos J, Guerri C, Rodríguez-Arias M, Miñarro J, Martínez-Riera R, Torrens M, Chowen JA, Argente J, Mason BJ, Pavón FJ, Rodríguez de Fonseca F (2015). Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment; influence of cocaine symptom severity and psychiatric co-morbidity. Addiction Biology, 20(4), 756–72. https://onlinelibrary.wiley.com/doi/10.1111/adb.12156 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

