Pharmaceutical, Clinical, and Regulatory Challenges of Reformulating Pressurized Metered-Dose Inhalers to Reduce Their Environmental Impact
- PMID: 39504952
- PMCID: PMC11839527
- DOI: 10.1089/jamp.2024.0023
Pharmaceutical, Clinical, and Regulatory Challenges of Reformulating Pressurized Metered-Dose Inhalers to Reduce Their Environmental Impact
Abstract
The chlorofluorocarbons (CFCs) that were used as propellants in early pressurized metered-dose inhalers (pMDIs) had substantial ozone-depleting potential. Following the Montreal Protocol in 1987, the manufacture of a range of ozone-depleting substances, including CFCs, was gradually phased out, which required the propellants used in pMDIs to be replaced. Current pMDIs use hydrofluoroalkanes (HFAs) as propellants, such as 1,1,1,2-tetrafluoroethane (HFA-134a). Although these HFAs have no ozone-depleting potential, they have a high global warming potential (GWP), and consequently, their use is being phased down. One option for the discontinuation of HFA use in inhalers would be to discontinue all pMDIs, switching patients to dry powder inhalers (DPIs). However, a switch from pMDIs to DPIs may not be a clinically appropriate option for some patients; furthermore, the full lifecycle carbon footprint and the overall environmental impact of different inhalers should be considered. An alternative is therefore to reformulate the current HFA pMDIs to use low-GWP propellants, such as 1,1-difluoroethane (HFA-152a). This article summarizes the various steps and challenges associated with this change, illustrated using data from the inhaled triple combination of beclomethasone dipropionate, formoterol fumarate, and glycopyrronium bromide, a complex formulation of three molecules in a solution that contains liquid-phase propellant.
Keywords: global warming; hydrofluoroalkanes; inhaler; ozone depletion.
Figures
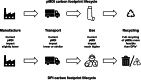
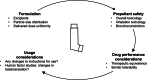




References
-
- United Nations Environment Programme. The Montreal Protocol on substances that Deplete the Ozone layer. 1987. Available from: https://ozone.unep.org/treaties/montreal-protocol [Last accessed: September 4, 2024].
-
- Leach C. The CFC to HFA transition and its impact on pulmonary drug development: Discussion. Respir Care 2005;50(9):1201–1206. - PubMed
-
- European Union. Regulation (EU) No 517/2014 of the European parliament and of the council of 16 april 2014 on fluorinated greenhouse gases and repealing regulation (EC) no 842/2006 text with EEA relevance. 2014. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.1... [Last accessed: May 4, 2024].
-
- United Nations Environment Programme. Amendment to the Montreal protocol on substances that deplete the Ozone layer. United Nations Environment Programme: Kigali, Rwanda; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
