The Novel Lipid Emulsion Vegaven Is Well Tolerated and Elicits Distinct Biological Actions Compared With a Mixed-Oil Lipid Emulsion Containing Fish Oil: A Parenteral Nutrition Trial in Piglets
- PMID: 39505265
- PMCID: PMC11934249
- DOI: 10.1016/j.tjnut.2024.10.047
The Novel Lipid Emulsion Vegaven Is Well Tolerated and Elicits Distinct Biological Actions Compared With a Mixed-Oil Lipid Emulsion Containing Fish Oil: A Parenteral Nutrition Trial in Piglets
Abstract
Background: Vegaven is a novel lipid emulsion for parenteral nutrition (PN) based on 18-carbon n-3 (ω-3) fatty acids, which elicits liver protection via interleukin-10 (IL-10) in the murine model of PN.
Objectives: In a preclinical model of PN in neonatal piglets, Vegaven was tested for efficacy and safety and compared with a mixed-oil lipid emulsion containing fish oil (SMOFlipid).
Methods: Male piglets 4-5 d old were randomly allocated to isocaloric isonitrogenous PN for 14 d, which varied only by the type of lipid emulsion (Vegaven, n = 8; SMOFlipid, n = 8). Hepatic IL-10 tissue concentration served as primary outcome. Secondary outcomes were organ weights, bile flow, blood analyses, plasma insulin and glucagon concentrations, insulin signaling, proinflammatory cytokines, tissue lipopolysaccharide concentrations, and fatty acid composition of phospholipid fractions in plasma, liver, and brain.
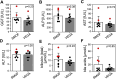
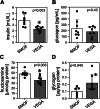
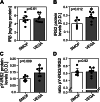
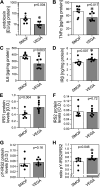
Results: Total weight gain on trial, organ weights, and bile flow were similar between the Vegaven and the SMOFlipid group. Vegaven elicited higher hepatic IL10 (Δ = 148 pg/mg protein; P < 0.001) and insulin receptor substrate-2 amounts (Δ = 0.08 OD; P = 0.012). Plasma insulin concentrations (Δ = 1.46 mU/L; P = 0.003) and fructosamine (glycated albumin, Δ = 12.4 μmol/g protein; P = 0.003) were increased in SMOFlipid as compared with those of Vegaven group, indicating insulin resistance. Higher hepatic injury markers were observed more frequently in the SMOFlipid group than those in the Vegaven group. Lipopolysaccharide, tumor necrosis factor-α, and IL-6 concentrations increased in pancreatic and brain tissues of SMOFlipid-treated compared with those in the Vegaven-treated piglets. Insulin signaling reduced in the brains of SMOFlipid-treated piglets. Vegaven and SMOFlipid elicited distinct fatty acid profiles in the phospholipid fractions of the rapidly growing brains but showed similar accretion of docosahexaenoic acid and arachidonic acid after 2 wk of PN.
Conclusions: Vegaven is well tolerated in this piglet model of PN, demonstrating distinct biological actions compared with SMOFlipid, namely lower liver, pancreas, and brain inflammation, enhanced insulin signaling, and improved whole body glucose control.
Keywords: 18-carbon n–3 fatty acids; hyperinsulinemia; insulin resistance; interleukin-10; lipid mediators; liver; mixed-oil lipid emulsions; neonatal piglet; neuroinflammation; pancreas; parenteral nutrition; stearidonic acid; α-linolenic acid.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest A patent application related to the novel lipid emulsion has been submitted to the European Patent Office (EP 21/204659.3). The authors report no conflicts of interest.
Figures



References
-
- Gura K.M., Calkins K.L., Premkumar M.H., Puder M. Use of intravenous soybean and fish oil emulsions in pediatric intestinal failure-associated liver disease: a multicenter integrated analysis report on extrahepatic adverse events. J. Pediatr. 2022;241:173–180.e1. doi: 10.1016/j.jpeds.2021.10.030. - DOI - PubMed
-
- Tran K., Butcher R. Lipid Formulations for Patients Requiring Parenteral Nutrition: A Review of Clinical Effectiveness. Cost-Effectiveness, and Guidelines—An Update, Canadian Agency for Drugs and Technologies in Health. August 2, 2019 Ottawa, ON, Canada. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

