Overcoming Copper Reduction Limitation in Asymmetric Substitution: Aryl-Radical-Enabled Enantioconvergent Cyanation of Alkyl Iodides
- PMID: 39505711
- PMCID: PMC11955248
- DOI: 10.1021/jacs.4c11888
Overcoming Copper Reduction Limitation in Asymmetric Substitution: Aryl-Radical-Enabled Enantioconvergent Cyanation of Alkyl Iodides
Abstract
Cu-catalyzed enantioconvergent cross-coupling of alkyl halides has emerged as a powerful strategy for synthesizing enantioenriched molecules. However, this approach is intrinsically limited by the weak reducing power of copper(I) species, which restricts the scope of compatible nucleophiles and necessitates extensive ligand optimization or the use of complex chiral scaffolds. To overcome these challenges, we introduce an aryl-radical-enabled strategy that decouples the alkyl halide activation step from the chiral Cu center. We demonstrate that merging aryl-radical-enabled iodine abstraction with Cu-catalyzed asymmetric radical functionalization enables the conversion of racemic α-iodoamides to enantioenriched alkyl nitrile products with good yield and enantioselectivity. The rational design of chiral ligands identified a new class of carboxamide-containing BOX ligands. Mechanistic studies support an aryl-radical-enabled pathway and the unique hydrogen-bonding ability in the newly designed BOX ligands. This aryl-radical-enabled asymmetric substitution reaction has the potential to significantly expand the scope of Cu-catalyzed enantioconvergent cross-coupling reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


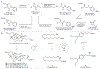
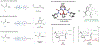
References
-
- Steinlandt PS; Zhang L; Meggers E Metal Stereogenicity in Asymmetric Transition Metal Catalysis. Chem. Rev 2023, 123 (8), 4764–4794. - PMC - PubMed
- Yoon TP; Jacobsen EN Privileged Chiral Catalysts. Science 2003, 299 (5613), 1691–1693. - PubMed
- List B Introduction: Organocatalysis. Chem. Rev 2007, 107 (12), 5413–5415.
- MacMillan DWC The advent and development of organocatalysis. Nature 2008, 455 (7211), 304–308. - PubMed
-
- Fu GC Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Cent. Sci 2017, 3 (7), 692–700. - PMC - PubMed
- Chen L-M; Reisman SE Enantioselective C(sp2)–C(sp3) Bond Construction by Ni Catalysis. Acc. Chem. Res 2024, 57 (5), 751–762. - PMC - PubMed
- Cherney AH; Kadunce NT; Reisman SE Enantioselective and Enantiospecific Transition-Metal-Catalyzed Cross-Coupling Reactions of Organometallic Reagents To Construct C–C Bonds. Chem. Rev 2015, 115 (17), 9587–9652. - PMC - PubMed
- Choi J; Fu GC Transition metal–catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356 (6334), No. eaaf7230. - PMC - PubMed
- Lipp A; Badir SO; Molander GA Stereoinduction in Metallaphotoredox Catalysis. Angew. Chem., Int. Ed 2021, 60 (4), 1714–1726. - PMC - PubMed
- Wang Z; Yin H; Fu GC Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 2018, 563 (7731), 379–383. - PMC - PubMed
-
- Tsarevsky NV; Matyjaszewski K Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev 2007, 107 (6), 2270–2299. - PubMed
- Matyjaszewski K; Tsarevsky NV Macromolecular Engineering by Atom Transfer Radical Polymerization. J. Am. Chem. Soc 2014, 136 (18), 6513–6533. - PubMed
- McCann SD; Stahl SS Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst. Acc. Chem. Res 2015, 48 (6), 1756–1766. - PubMed
- Hossain A; Bhattacharyya A; Reiser O Copper’s rapid ascent in visible-light photoredox catalysis. Science 2019, 364 (6439), No. eaav9713. - PubMed
- Thapa S; Shrestha B; Gurung SK; Giri R Copper-catalysed cross-coupling: an untapped potential. Org. Biomol. Chem 2015, 13 (17), 4816–4827. - PubMed
- Trammell R; Rajabimoghadam K; Garcia-Bosch I Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2Model Systems to Organometallic Transformations. Chem. Rev 2019, 119 (4), 2954–3031. - PMC - PubMed
- Egorova KS; Ananikov VP Which Metals are Green for Catalysis? Comparison of the Toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au Salts. Angew. Chem., Int. Ed 2016, 55 (40), 12150–12162. - PubMed
- Egorova KS; Ananikov VP Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36 (21), 4071–4090.
- Cheng L-J; Mankad NP C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev 2020, 49 (22), 8036–8064. - PubMed
-
- Hickman AJ; Sanford MS High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185. - PMC - PubMed
- Casitas A; King AE; Parella T; Costas M; Stahl SS; Ribas X Direct observation of CuI/CuIII redox steps relevant to Ullmann-type coupling reactions. Chem. Sci 2010, 1 (3), 326–330.
- Casitas A; Canta M; Solà M; Costas M; Ribas X Nucleophilic Aryl Fluorination and Aryl Halide Exchange Mediated by a CuI/CuIII Catalytic Cycle. J. Am. Chem. Soc 2011, 133 (48), 19386–19392. - PubMed
- Liu S; Liu H; Liu S; Lu Z; Lu C; Leng X; Lan Y; Shen Q C(sp3)-CF3 Reductive Elimination from a Five-Coordinate Neutral Copper(III) Complex. J. Am. Chem. Soc 2020, 142 (21), 9785–9791. - PubMed
- Wang G; Li M; Leng X; Xue X; Shen Q Neutral Five-Coordinate Arylated Copper(III) Complex: Key Intermediate in Copper-Mediated Arene Trifluoromethylation. Chin. J. Chem 2022, 40 (16), 1924–1930.
- Lu Z; Liu H; Liu S; Leng X; Lan Y; Shen Q A Key Intermediate in Copper-Mediated Arene Trifluoromethylation, [nBu4N][Cu(Ar)(CF3)3]: Synthesis, Characterization, and C(sp2)–CF3 Reductive Elimination. Angew. Chem., Int. Ed 2019, 58 (25), 8510–8514. - PubMed
- Liu H; Shen Q Well-defined organometallic Copper(III) complexes: Preparation, characterization and reactivity. Coord. Chem. Rev 2021, 442, No. 213923.
- Liu L; Xi Z Organocopper (III) Compounds with Well-defined Structures Undergo Reductive Elimination to Form C—C or C–Heteroatom Bonds. Chin. J. Chem 2018, 36 (12), 1213–1221.
- Liu L; Zhu M; Yu H-T; Zhang W-X; Xi Z Organocopper(III) Spiro Complexes: Synthesis, Structural Characterization, and Redox Transformation. J. Am. Chem. Soc 2017, 139 (39), 13688–13691. - PubMed
- Paeth M; Tyndall SB; Chen L-Y; Hong J-C; Carson WP; Liu X; Sun X; Liu J; Yang K; Hale EM; Tierney DL; Liu B; Cao Z; Cheng M-J; Goddard WA; Liu W Csp3–Csp3 Bond-Forming Reductive Elimination from Well-Defined Copper(III) Complexes. J. Am. Chem. Soc 2019, 141 (7), 3153–3159. - PubMed
- Blythe IM; Xu J; Odell JSF; Kampf JW; Bowring MA; Sanford MS Characterization and Reactivity of Copper(II) and Copper(III) σ-Aryl Intermediates in Aminoquinoline-Directed C–H Functionalization. J. Am. Chem. Soc 2023, 145 (33), 18253–18259. - PubMed
- Reese MS; Bonanno MG; Bower JK; Moore CE; Zhang S C–N Bond Formation at Discrete CuIII–Aryl Complexes. J. Am. Chem. Soc 2023, 145 (49), 26810–26816. - PMC - PubMed
- Luo Y; Li Y; Wu J; Xue X-S; Hartwig JF; Shen Q Oxidative addition of an alkyl halide to form a stable Cu(III) product. Science 2023, 381 (6662), 1072–1079. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

