Constructing organoid-brain-computer interfaces for neurofunctional repair after brain injury
- PMID: 39505863
- PMCID: PMC11541701
- DOI: 10.1038/s41467-024-53858-2
Constructing organoid-brain-computer interfaces for neurofunctional repair after brain injury
Abstract
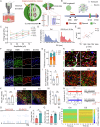
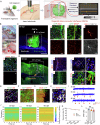
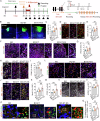
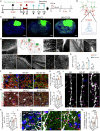
The reconstruction of damaged neural circuits is critical for neurological repair after brain injury. Classical brain-computer interfaces (BCIs) allow direct communication between the brain and external controllers to compensate for lost functions. Importantly, there is increasing potential for generalized BCIs to input information into the brains to restore damage, but their effectiveness is limited when a large injured cavity is caused. Notably, it might be overcome by transplantation of brain organoids into the damaged region. Here, we construct innovative BCIs mediated by implantable organoids, coined as organoid-brain-computer interfaces (OBCIs). We assess the prolonged safety and feasibility of the OBCIs, and explore neuroregulatory strategies. OBCI stimulation promotes progressive differentiation of grafts and enhances structural-functional connections within organoids and the host brain, promising to repair the damaged brain via regenerating and regulating, potentially directing neurons to preselected targets and recovering functional neural networks in the future.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

