Nucleotidyltransferase toxin MenT extends aminoacyl acceptor ends of serine tRNAs to control Mycobacterium tuberculosis growth
- PMID: 39505885
- PMCID: PMC11541572
- DOI: 10.1038/s41467-024-53931-w
Nucleotidyltransferase toxin MenT extends aminoacyl acceptor ends of serine tRNAs to control Mycobacterium tuberculosis growth
Abstract
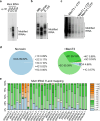
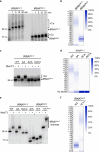
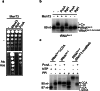
Toxins of toxin-antitoxin systems use diverse mechanisms to inhibit bacterial growth. In this study, we characterize the translation inhibitor toxin MenT3 of Mycobacterium tuberculosis, the bacterium responsible for tuberculosis in humans. We show that MenT3 is a robust cytidine specific tRNA nucleotidyltransferase in vitro, capable of modifying the aminoacyl acceptor ends of most tRNA but with a marked preference for tRNASer, to which long stretches of cytidines are added. Furthermore, transcriptomic-wide analysis of MenT3 targets in M. tuberculosis identifies tRNASer as the sole target of MenT3 and reveals significant detoxification attempts by the essential CCA-adding enzyme PcnA in response to MenT3. Finally, under physiological conditions, only in the presence the native menAT3 operon, an active pool of endogenous MenT3 targeting tRNASer in M. tuberculosis is detected, likely reflecting the importance of MenT3 during infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Harms, A., Brodersen, D. E., Mitarai, N. & Gerdes, K. Toxins, targets, and triggers: an overview of toxin-antitoxin biology. Mol. Cell70, 768–784 (2018). - PubMed
-
- Jurėnas, D., Fraikin, N., Goormaghtigh, F. & Van Melderen, L. Biology and evolution of bacterial toxin-antitoxin systems. Nat. Rev. Microbiol.10.1038/s41579-021-00661-1 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EQU202403018015/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- ANR-20-PAMR-0005/Agence Nationale de la Recherche (French National Research Agency)
- CRSII3_160703/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

