Magnetic resonance-conditional cardiac implantable electronic devices: an Italian perspective on the prevalence of mixed-brand systems over time
- PMID: 39505923
- PMCID: PMC11541881
- DOI: 10.1038/s41598-024-73403-x
Magnetic resonance-conditional cardiac implantable electronic devices: an Italian perspective on the prevalence of mixed-brand systems over time
Abstract
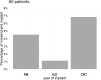
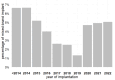
The historical restriction of magnetic resonance imaging (MRI) for patients with cardiac implantable electronic devices (CIEDs) has been lifted by certified MRI-conditional systems in recent years. Mixed-brand CIED systems consisting of a generator from one manufacturer and at least one lead from another manufacturer are not certified for MRI. We evaluated the temporal trend in the prevalence of mixed-brand systems in the era of MRI-conditional systems. Data were analyzed on 5853 CIEDs implanted de novo between 2012 and 2022 in 81 Italian centers linked to the nationwide Home Monitoring Expert Alliance network. The percentage of mixed-brand implants was calculated by device type (pacemaker, implantable cardioverter-defibrillator [ICD], cardiac resynchronization therapy [CRT] device) and over time. A mixed-brand system was implanted in 4.1% (95% CI, 3.6-4.6%) of analyzed patients or, by device type, in 4.5% (3.5-5.7%) of pacemaker patients, 1.1% (0.7-1.7%) of ICD patients, and 6.8% (5.7-7.9%) of CRT pacemaker/defibrillator patients (p < 0.001). Prevalence of mixed-brand implants exhibited significant temporal fluctuations, first declining from 6.6% (2012-2014) to 1.3% (2019), and then increasing to 5.1% (2022). Temporal changes were statistically significant for pacemakers (p < 0.001) and CRT devices (p = 0.001), but not for ICDs (p = 0.438). In the decade between 2012 and 2022, mixed-brand CIED systems were more prevalent in patients treated with pacemakers and CRT devices than in ICD recipients. A decline in the prevalence of mixed-brand systems was observed after the introduction of MRI-conditional systems, reaching a minimum in 2019, followed by a progressive increase in the subsequent years.
Keywords: Cardiac implantable electronic devices; Implantable cardioverter-defibrillator; MRI-conditional; Magnetic resonance imaging; Pacemaker.
© 2024. The Author(s).
Conflict of interest statement
Irene Baldasserre, Daniele Giacopelli and Alessio Gargaro are employees of BIOTRONIK Italia.
Figures

References
-
- Biffi, M. et al. Manufacturer change and risk of system-related complications after implantable cardioverter defibrillator replacement: physicians’ survey and data from the Detect Long-Term complications after Implantable Cardioverter Defibrillator replacement Registry. J. Cardiovasc. Med. (Hagerstown). 18 (12), 968–975 (2017). - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

