Multi-task Bayesian model combining FDG-PET/CT imaging and clinical data for interpretable high-grade prostate cancer prognosis
- PMID: 39505979
- PMCID: PMC11541986
- DOI: 10.1038/s41598-024-77498-0
Multi-task Bayesian model combining FDG-PET/CT imaging and clinical data for interpretable high-grade prostate cancer prognosis
Abstract
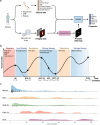
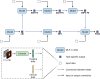
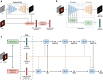
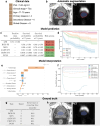
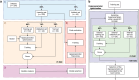
We propose a fully automatic multi-task Bayesian model, named Bayesian Sequential Network (BSN), for predicting high-grade (Gleason 8) prostate cancer (PCa) prognosis using pre-prostatectomy FDG-PET/CT images and clinical data. BSN performs one classification task and five survival tasks: predicting lymph node invasion (LNI), biochemical recurrence-free survival (BCR-FS), metastasis-free survival, definitive androgen deprivation therapy-free survival, castration-resistant PCa-free survival, and PCa-specific survival (PCSS). Experiments are conducted using a dataset of 295 patients. BSN outperforms widely used nomograms on all tasks except PCSS, leveraging multi-task learning and imaging data. BSN also provides automated prostate segmentation, uncertainty quantification, personalized feature-based explanations, and introduces dynamic predictions, a novel approach that relies on short-term outcomes to refine long-term prognosis. Overall, BSN shows great promise in its ability to exploit imaging and clinicopathological data to predict poor outcome patients that need treatment intensification with loco-regional or systemic adjuvant therapy for high-risk PCa.
Keywords: Bayesian; FDG-PET/CT; Multi-modal; Multi-task; Prognosis; Prostate cancer; Segmentation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

