Deep functional measurements of Fragile X syndrome human neurons reveal multiparametric electrophysiological disease phenotype
- PMID: 39506078
- PMCID: PMC11541539
- DOI: 10.1038/s42003-024-07120-6
Deep functional measurements of Fragile X syndrome human neurons reveal multiparametric electrophysiological disease phenotype
Abstract
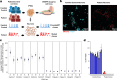
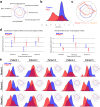
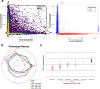
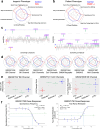
Fragile X syndrome (FXS) is a neurodevelopmental disorder caused by hypermethylation of expanded CGG repeats (>200) in the FMR1 gene leading to gene silencing and loss of Fragile X Messenger Ribonucleoprotein (FMRP) expression. FMRP plays important roles in neuronal function, and loss of FMRP in mouse and human FXS cell models leads to aberrant synaptic signaling and hyperexcitability. Multiple drug candidates have advanced into clinical trials for FXS, but no efficacious treatment has been identified to date, possibly as a consequence of poor translation from pre-clinical animal models to human. Here, we use a high resolution all-optical electrophysiology platform applied to multiple FXS patient-derived and CRISPR/Cas9-generated isogenic neuronal cell lines to develop a multi-parametric FXS disease phenotype. This neurophysiological phenotype was optimized and validated into a high throughput assay based on the amount of FMRP re-expression and the number of healthy neurons in a mosaic network necessary for functional rescue. The resulting highly sensitive and multiparameter functional assay can now be applied as a discovery platform to explore new therapeutic approaches for the treatment of FXS.
© 2024. The Author(s).
Conflict of interest statement
J.J.F., K.H., S.J.R., J.J., J.G., L.A.W., and G.T.D. are current employees of Quiver Bioscience and may hold stock options in Quiver Bioscience. D.G. was an employee of Q-State Bioscience, is currently a consultant for Quiver Bioscience and has received stock options in Quiver Bioscience. E.N., C.F., M.B., B.F. are current employees of Vertex Pharmaceuticals Inc. and hold stock in Vertex Pharmaceuticals Inc. N.D.B. is a former employee of Q-State Biosciences and may be an inventor on relevant patents, but holds no financial stake, stock, or options. M.F.B. is a co-founder of Allos Pharma, and a consultant and owner of stock options for Addex Therapeutics and Luminopia. H.U. and H.S. are current employees of Wave Life Sciences and report stock ownership in Wave Life Sciences. R.P.D. was employed at Vertex Pharmaceuticals at the time of the study, is a current employee of Intellia Therapeutics, and reports stock ownership in Intellia Therapeutics. Elizabeth Berry-Kravis has received funding from Acadia, Alcobra, AMO, Asuragen, Avexis, Biogen, BioMarin, Cydan, Engrail, Erydel, Fulcrum, GeneTx, GW, Healx, Ionis, Jaguar, Kisbee, Lumos, Marinus, Moment Biosciences, Neuren, Neurogene, Neurotrope, Novartis, Orphazyme/Kempharm, Ovid, PTC Therapeutics, Retrophin, Roche, Seaside Therapeutics, Taysha, Tetra, Ultragenyx, Yamo, Zynerba, and Vtesse/Sucampo/Mallinckrodt Pharmaceuticals, to consult on trial design or run clinical or lab validation trials in genetic neurodevelopmental or neurodegenerative disorders, all of which is directed to RUMC in support of rare disease programs; B.-K. receives no personal funds and RUMC has no relevant financial interest in any of the commercial entities listed.
Figures

References
-
- Penagarikano, O., Mulle, J. G. & Warren, S. T. The Pathophysiology of Fragile X Syndrome. Annual Review of Genomics and Human Genetics, 10.1146/annurev.genom.8.080706.092249 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

