A novel approach to engineering three-dimensional bladder tumor models for drug testing
- PMID: 39506094
- PMCID: PMC11542063
- DOI: 10.1038/s41598-024-78440-0
A novel approach to engineering three-dimensional bladder tumor models for drug testing
Abstract
Bladder cancer (BCa) poses a significant health challenge, particularly affecting men with higher incidence and mortality rates. Addressing the need for improved predictive models in BCa treatment, this study introduces an innovative 3D in vitro patient-derived bladder cancer tumor model, utilizing decellularized pig bladders as scaffolds. Traditional 2D cell cultures, insufficient in replicating tumor microenvironments, have driven the development of sophisticated 3D models. The study successfully achieved pig bladder decellularization through multiple cycles of immersion in salt solutions, resulting in notable macroscopic and histological changes. This process confirmed the removal of cellular components while preserving the native extracellular matrix (ECM). Quantitative analysis demonstrated the efficacy of decellularization, with a remarkable reduction in DNA concentration, signifying the removal of over 95% of cellular material. In the development of the in vitro bladder cancer model, muscle invasive bladder cancer patients' cells were cultured within decellularized pig bladders, yielding a three-dimensional cancer model. Optimal results were attained using an air-liquid interface technique, with cells injected directly into the scaffold at three distinct time points. Histological evaluations showcased characteristics resembling in vivo tumors derived from bladder cancer patients' cells. To demonstrate the 3D cancer model's effectiveness as a drug screening platform, the study treated it with Cisplatin (Cis), Gemcitabine (Gem), and a combination of both drugs. Comprehensive cell viability assays and histological analyses illustrated changes in cell survival and proliferation. The model exhibited promising correlations with clinical outcomes, boasting an 83.3% reliability rate in predicting treatment responses. Comparison with traditional 2D cultures and spheroids underscored the 3D model's superiority in reliability, with an 83.3% predictive capacity compared to 50% for spheroids and 33.3% for 2D culture. Acknowledging limitations, such as the absence of immune and stromal components, the study suggests avenues for future improvements. In conclusion, this innovative 3D bladder cancer model, combining decellularization and patient-derived cells, marks a significant advancement in preclinical drug testing. Its potential for predicting treatment outcomes and capturing patient-specific responses opens new avenues for personalized medicine in bladder cancer therapeutics. Future refinements and validations with larger patient cohorts hold promise for revolutionizing BCa research and treatment strategies.
Keywords: Bladder cancer; Cancer model; Decellularization; Recellularization; Tissue engineering.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




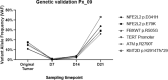

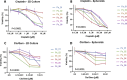
References
-
- Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2023. Toronto, ON: Canadian Cancer Society; 2023. Available at: cancer.ca/Canadian-Cancer-Statistics-2023-EN
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

