Nucleosome flipping drives kinetic proofreading and processivity by SWR1
- PMID: 39506114
- PMCID: PMC11618073
- DOI: 10.1038/s41586-024-08152-y
Nucleosome flipping drives kinetic proofreading and processivity by SWR1
Abstract
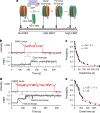
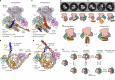
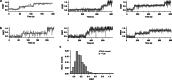
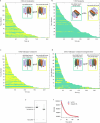
The yeast SWR1 complex catalyses the exchange of histone H2A-H2B dimers in nucleosomes, with Htz1-H2B dimers1-3. Here we used single-molecule analysis to demonstrate two-step double exchange of the two H2A-H2B dimers in a canonical yeast nucleosome with Htz1-H2B dimers, and showed that double exchange can be processive without release of the nucleosome from the SWR1 complex. Further analysis showed that bound nucleosomes flip between two states, with each presenting a different face, and hence histone dimer, to SWR1. The bound dwell time is longer when an H2A-H2B dimer is presented for exchange than when presented with an Htz1-H2B dimer. A hexasome intermediate in the reaction is bound to the SWR1 complex in a single orientation with the 'empty' site presented for dimer insertion. Cryo-electron microscopy analysis revealed different populations of complexes showing nucleosomes caught 'flipping' between different conformations without release, each placing a different dimer into position for exchange, with the Swc2 subunit having a key role in this process. Together, the data reveal a processive mechanism for double dimer exchange that explains how SWR1 can 'proofread' the dimer identities within nucleosomes.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
-
- Krogan, N. J. et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell12, 1565–1576 (2003). - PubMed
-
- Mizuguchi, G. et al. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science303, 343–348 (2004). - PubMed
-
- Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature389, 251–260 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

