Pharmacokinetics and brain uptake of sodium selenate and selenium in naïve rats and a lateral fluid percussion injury rat model
- PMID: 39506350
- PMCID: PMC11540874
- DOI: 10.1002/prp2.1256
Pharmacokinetics and brain uptake of sodium selenate and selenium in naïve rats and a lateral fluid percussion injury rat model
Abstract
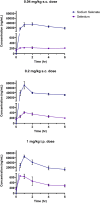
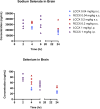
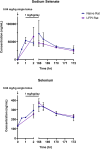
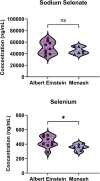
Post-traumatic epilepsy (PTE) is a life-long complication of traumatic brain injury (TBI). The development of PTE is associated with neurological morbidity and increases the risk of mortality. An aim of EpiBioS4Rx (Epilepsy Bioinformatics Study for Antiepileptogenic Therapy) was to test potential therapies to prevent the development of PTE in the lateral fluid percussion injury (LFPI) rat model of TBI, in which rats were subjected to injury at the left parietal cortex. Sodium selenate has been reported to be antiepileptogenic post-TBI in rodent models by activating protein phosphatase 2A and reducing phosphorylated tau (p-tau) protein. We aimed to characterize the pharmacokinetics (PK) and brain uptake of sodium selenate using naïve control and LFPI rats. Rats received either a single bolus dose or a single bolus dose followed by a 7-day subcutaneous minipump infusion of sodium selenate. Sodium selenate and selenium concentrations in plasma and brain were analyzed and used for PK estimation and brain exposure assessment. Selenium concentrations rapidly increased after sodium selenate administration, demonstrating biotransformation from sodium selenate to selenium. Sodium selenate and selenium PK parameters were estimated using non-compartmental analysis. Sodium selenate clearance (CL/F) and volume of distribution (Vd/F) varied by dose and route of administration, suggesting differences in bioavailability and nonlinear pharmacokinetics at the doses tested. Brain-to-plasma partition coefficients (AUCbrain/AUCplasma) for sodium selenate and selenium were found to be 0.7-1.3 and 0.1-0.3 following single-dose injection, respectively, indicating active transport of sodium selenate across the blood-brain barrier (BBB).
Keywords: antiepileptogenesis; blood–brain barrier; pharmacokinetics; post‐traumatic epilepsy; traumatic brain injury.
© 2024 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
SL Moshé is the Charles Frost Chair in Neurosurgery and Neurology and partially funded by grants from NIH U54 NS100064 (EpiBioS4Rx), RO1‐NS43209 and RO1‐NS127524, US Department of Defense (W81XWH‐22‐1‐0510, W81XWH‐22‐1‐0210), a pilot grant from the NICHD center grant (P50 HD105352) for the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK‐IDDRC), and the Heffer Family and the Segal Family Foundations, the Isabelle Rapin and Harold Oaklander Child Neurology Research Fund in the Isabelle Rapin Child Neurology Division, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. He is on the editorial board of Brain and Development, Pediatric Neurology, Annals of Neurology, MedLink, and Physiological Research. He receives compensation from MedLink for his work as Associate Editor; and royalties from books he co‐edited. AS Galanopoulou received grant support from the NINDS U54 NS100064 (EpiBioS4Rx), NINDS R01 NS127524, a pilot grant from NICHD center grant (P50 HD105352) for the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK‐IDDRC), US Department of Defense (W81XWH‐22‐1‐0510, W81XWH‐22‐1‐0210), the Heffer Family and the Segal Family Foundations, the Isabelle Rapin and Harold Oaklander Child Neurology Research Fund in the Isabelle Rapin Child Neurology Division, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. AS Galanopoulou is the Editor‐in‐Chief of
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

