Gamma-irradiated copper-based metal organic framework nanocomposites for photocatalytic degradation of water pollutants and disinfection of some pathogenic bacteria and fungi
- PMID: 39506685
- PMCID: PMC11539452
- DOI: 10.1186/s12866-024-03587-9
Gamma-irradiated copper-based metal organic framework nanocomposites for photocatalytic degradation of water pollutants and disinfection of some pathogenic bacteria and fungi
Abstract
Background: Although there are many uses for metal-organic framework (MOF) based nanocomposites, research shows that these materials have received a lot of interest in the field of water treatment, namely in the photodegradation of water contaminants, and disinfection of some pathogenic bacteria and fungi. This is brought on by excessive water pollution, a lack of available water, low-quality drinking water, and the emergence of persistent micro-pollutants in water bodies. Photocatalytic methods may be used to remove most water contaminants, and pathogenic microbes, and MOF is an excellent modifying and supporting material for photocatalytic degradation.
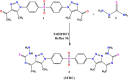
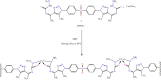
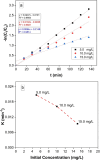
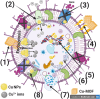
Methods: This work involved the fabrication of a unique Cu-MOF based nanocomposite that was exposed to gamma radiation. The nanocomposite was subsequently employed for photocatalytic degradation and as an antimicrobial agent against certain harmful bacteria and fungi. The produced Cu-MOf nanocomposite was identified by XRD, SEM, and EDX. Growth curve analysis, UV lighting impact, and antibiofilm potential have been carried out to check antimicrobial potential. Additionally, the membrane leakage test was used to determine the mechanism of the antimicrobial action. In an experimental investigation of photocatalytic activity, a 50 mL aqueous solution including 10.0 ppm of Rhodamine B (RB) was used to solubilize 10 mg of Cu-MOF. It has been investigated how pH and starting concentration affect RB elimination by Cu-MOF. Ultimately, RB elimination mechanism and kinetic investigations have been carried out.
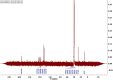
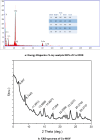
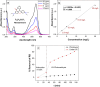
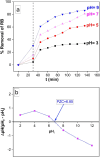
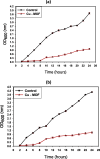
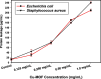
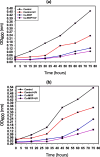
Results: SEM images from the characterization techniques demonstrated the fact that the Cu-MOF was synthesized effectively and exhibited the Cu-MOF layers' flake-like form. Uneven clusters of rods make up each stratum. The primary peaks in the Cu-MOF's diffraction pattern were found at 2θ values of 8.75◦, 14.83◦, 17.75◦, 21.04◦, 22.17◦, 23.31◦, 25.41◦, and 26.38◦, according to the XRD data. After 135 min of UV irradiation, only 8% of RB had undergone photolytic destruction. On the other hand, the elimination resulting from adsorption during a 30-min period without light was around 16%. Conversely, after 135 min, Cu-MOF's photocatalytic breakdown of RB with UV light reached 81.3%. At pH 9.0, the greatest removal of RB at equilibrium was found, and when the amount of photocatalyst rose from 5 to 20 mg, the removal efficiency improved as well. The most sensitive organism to the synthesized Cu-MOF, according to antimicrobial data, was Candida albicans, with a documented MIC value of 62.5 µg mL-1 and antibacterial ZOI as 32.5 mm after 1000 ppm treatment. Cu-MOF also showed the same MIC (62.5 µg mL-1) values against Staphylococcus aureus and Escherichia coli, and 35.0 and 32.0 mm ZOI after 1000 ppm treatment, respectively. Ultimately, it was found that Cu-MOF (1000 µg/mL) after having undergone gamma irradiation (100.0 kGy) was more effective against S. aureus (42.5 mm ZOI) and E. coli (38.0 mm ZOI).
Conclusion: From the obtained results, the synthesized MOF nanocomposites had promising catalytic degradation of RB dye and high antimicrobial potential which encouraging their use in wastewater treatment against some pathogenic microbes and polluted dyes. Due to the exceptional physicochemical characteristics of MOF nanocomposites, it is possible to create and modify photocatalytic nanocomposites in a way that improves their recovery, efficiency, and recyclability.
Keywords: 1, 2, 3-triazoles; Antimicrobial activity; Cu-MOFs; Gamma-rays; Nanocomposites; Photocatalytic degradation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

