Dynamics of cell-free tumor DNA correlate with early MRI response during chemoradiotherapy in rectal cancer
- PMID: 39506775
- PMCID: PMC11539469
- DOI: 10.1186/s13014-024-02540-4
Dynamics of cell-free tumor DNA correlate with early MRI response during chemoradiotherapy in rectal cancer
Abstract
Background: In locally advanced rectal cancer, the prediction of tumor response during and after neoadjuvant treatment remains challenging. In terms of organ preservation, adaptive radiotherapy, and intensified (total) neoadjuvant therapies, biomarkers are desirable for patient stratification.
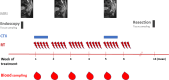
Methods: In 16 patients, weekly blood samples (n = 86) to detect cell-free tumor DNA (ctDNA) during long-course neoadjuvant chemoradiotherapy were analyzed. Data were correlated with initial tumor volumes, MRI response in week 2 and 5 of radiotherapy as well as with pathologic tumor response after resection and outcome parameters.
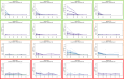
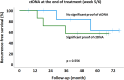
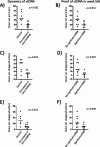
Results: Most patients showed decreasing ctDNA during the course of radiochemotherapy. However, we found heterogenous dynamics of ctDNA and could identify three groups: (1) decline (2) no clear decline and/or late shedding (3) persistence of ctDNA. In seven patients we could detect significant amounts of ctDNA in week 5 or week 6 of treatment. In our pilot cohort, we did not find significant correlations of ctDNA dynamics with pathologic response or outcome parameters. However, patients with distinct decline of ctDNA had larger tumor volumes prior to treatment, and MRI imaging in week 2 and 5 revealed bigger absolute decrease of tumor volumes. If significant levels of ctDNA were found in week 5 and / or 6, patients showed less absolute tumor volume decrease in week 2 and 5.
Conclusions: Weekly measurement of ctDNA during radiochemotherapy is feasible and might represent a promising biomarker. Bigger initial primary tumors showed different ctDNA shedding profiles compared with smaller primary tumors and correlations of ctDNA dynamics with early imaging response were found.
Keywords: Adaptive radiotherapy; Biomarker; Imaging; Magnetic resonance imaging; NGS; cfDNA; ctDNA.
© 2024. The Author(s).
Conflict of interest statement
KC, CG and MN report institutional collaborations including financial and non-financial support by Elekta, Philips, Siemens, Dr. Sennewald, PTW Freiburg, Kaiku and Therapanacea. CG reports honoraria and travel support from Elekta outside this work. CS reports institutional grants from Novartis and Illumina as well as research grants from BMS Stiftung Immunonkologie outside the submitted work.
Figures
References
-
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33. - DOI - PubMed
-
- Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–89. - DOI - PubMed
-
- Conroy T, Bosset JF, Etienne PL, Rio E, Francois E, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–15. - DOI - PubMed
-
- Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

