Azithromycin in severe malaria bacterial co-infection in African children (TABS-PKPD): a phase II randomised controlled trial
- PMID: 39506794
- PMCID: PMC11542398
- DOI: 10.1186/s12916-024-03712-5
Azithromycin in severe malaria bacterial co-infection in African children (TABS-PKPD): a phase II randomised controlled trial
Abstract
Background: African children with severe malaria are at increased risk of non-typhoidal salmonellae co-infection. Broad-spectrum antibiotics are recommended by guidelines but the optimal class and dose have not been established. We investigated the optimal dose of oral dispersible azithromycin and whether simple clinical criteria and point-of-care biomarkers could target antibiotics to those at greatest risk of bacterial co-infection.
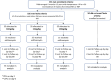
Methods: We conducted a phase I/II trial in Ugandan children with severe malaria comparing a 5-day course of azithromycin: 10, 15 and 20 mg/kg of azithromycin (prescribed by weight bands) spanning the dose-range effective for other salmonellae infection. We generated relevant pharmacokinetic (PK) data by sparse sampling during dosing intervals and investigated associations between azithromycin exposure and potential mechanisms (PK-pharmacodynamics) using change in C-reactive protein (CRP), a putative marker of sepsis, at 72 h (continuous) and microbiological cure (7-day) (binary), alone and as a composite with 7-day and 90-day survival. To assess whether clinical or biomarkers could identify those at risk of sepsis, a non-severe malaria control was concurrently enrolled.
Results: Between January 2020 and January 2022, 105 cases were randomised azithromycin doses: 35 to 10 mg/kg, 35 to 15 mg/kg and 35 to 20 mg/kg. Fifty non-severe malaria controls were concurrently enrolled. CRP reduced in all arms by 72 h with a mean reduction of 65.8 mg/L (95% CI 57.1, 74.5) in the 10 mg/kg arm, 64.8 mg/L (95% CI 56.5, 73.1; p = 0.87) in the 20 mg/kg arm and a smaller reduction 51.2 mg/L (95% CI 42.9, 59.5; p = 0.02) in the 15 mg/kg arm. Microbiological cure alone outcome was not analysed as only one pathogen was found among cases. Three events contributed to the composite outcome of 7-day survival and microbiological cure, with no events in the 15 mg/kg arm. The odds ratio comparing 20 vs 10 mg/kg was 0.50 (95% CI 0.04, 5.79); p = 0.58. Due to the low number of pathogens identified, it was not possible to identify better methods for targeting antibiotics including both the cases and controls.
Conclusions: We found no evidence for an association between systemic azithromycin exposure and reduction in CRP. Further work is needed to better identify children at highest risk from bacterial co-infection.
Trial registration: ISRCTN49726849 (registered on 27th October 2017).
Keywords: African children; Bacterial infection; Clinical trial; Pharmacokinetics; Severe malaria.
© 2024. The Author(s).
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


References
-
- World malaria report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
-
- World Health Organization. Guidelines for the treatment of malaria. World Health Organization; 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

