Drug-coated balloons versus drug-eluting stents in patients with in-stent restenosis: An updated meta-analysis with trial sequential analysis
- PMID: 39506808
- PMCID: PMC11539716
- DOI: 10.1186/s13019-024-03046-6
Drug-coated balloons versus drug-eluting stents in patients with in-stent restenosis: An updated meta-analysis with trial sequential analysis
Abstract
Background: Drug-coated balloons (DCB) have promising results in the management of in-stent restenosis (ISR), still their role remains a major challenge, and not well established in contemporary clinical practice.
Aims: To provide a comprehensive appraisal of the efficacy and safety of DCBs in patients with in-stent restenosis (ISR).
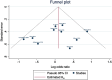
Methods: We searched PubMed, Scopus, web of Science, Ovid, and Cochrane Central from inception until 30 March, 2023. We included randomized controlled trials (RCTs) that compared DCB versus DES in ISR patients. Our primary endpoints were major adverse cardiac events (MACE) and late lumen loss (LLL). Secondary clinical endpoints were all-cause death, cardiac death, MI, TLR, TVR, and stent thrombosis, and angiographic outcomes were MLD, and in-stent binary restenosis.
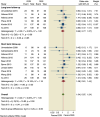
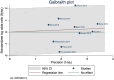
Results: Ten RCTs comprising 1977 patients were included in this meta-analysis. The incidence of MACE was 15.57% in the DCB group compared to 14.13% in the DES group, with no significant difference in the risk of MACE following DCB (odds ratio [OR] 1.04, 95% confidence interval [CI]: 0.87 to 1.44). Compared with the DES intervention, the risk of LLL was comparable to the DCB intervention (mean difference [MD] -0.08, 95% CI: -0.18 to 0.02), while the incidence of TLR was increased in the DCB intervention (OR: 1.54, 95% CI: 1.2 to 1.99).
Conclusion: DCB was comparable to DES implantation is ISR patients regarding clinical outcomes, however it showed an increase in TLR events. Moreover, a RCT with large sample size and longer follow-up duration is warrened to validate these results.
Keywords: DCB; DES; In-stent restenosis; Meta-analysis; PCI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Habara S, Iwabuchi M, Inoue N, Nakamura S, Asano R, Nanto S, et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am Heart J. 2013;166:527–e5332. 10.1016/j.ahj.2013.07.002. - DOI - PubMed
-
- Jensen CJ, Richardt G, Tölg R, Erglis A, Skurk C, Jung W, et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroIntervention. 2018;14:1096–103. 10.4244/EIJ-D-17-01079. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

