Etrinabdione (VCE-004.8), a B55α activator, promotes angiogenesis and arteriogenesis in critical limb ischemia
- PMID: 39506809
- PMCID: PMC11539538
- DOI: 10.1186/s12967-024-05748-w
Etrinabdione (VCE-004.8), a B55α activator, promotes angiogenesis and arteriogenesis in critical limb ischemia
Abstract
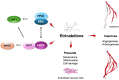
Background: Vasculogenic therapies explored for the treatment of peripheral artery disease (PAD) have encountered minimal success in clinical trials. Addressing this, B55α, an isoform of protein phosphatase 2A (PP2A), emerges as pivotal in vessel remodeling through activation of hypoxia-inducible factor 1α (HIF-1α). This study delves into the pharmacological profile of VCE-004.8 (Etrinabdione) and evaluates its efficacy in a preclinical model of critical limb ischemia, with a focus on its potential as a PP2A/B55α activator to induce angiogenesis and arteriogenesis.
Methods: Vascular endothelial cells were used for in vitro experiments. Aorta ring assay was performed to explore sprouting activity. Matrigel plug-in assay was used to assess the angiogenic potential. Critical limb ischemia (CLI) in mice was induced by double ligation in the femoral arteria. Endothelial vascular and fibrotic biomarkers were studied by immunohistochemistry and qPCR. Arteriogenesis was investigated by microvascular casting and micro-CT. Proteomic analysis in vascular tissues was analyzed by LC-MS/MS. Ex-vivo expression of B55α and biomarkers were investigated in artery samples from PAD patients.
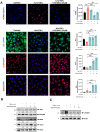
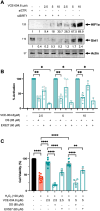
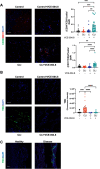
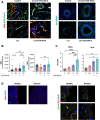
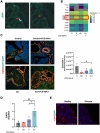
Results: VCE-004.8 exhibited the ability to induce B55α expression and activate the intersecting pathways B55α/AMPK/Sirtuin 1/eNOS and B55α/PHD2/HIF-1α. VCE-004.8 prevented OxLDL and H2O2-induced cytotoxicity, senescence, and inflammation in endothelial cells. Oral VCE-004.8 increased aorta sprouting in vitro and angiogenesis in vivo. In CLI mice VCE-004.8 improved collateral vessel formation and induced endothelial cells proliferation, angiogenic gene expression and prevented fibrosis. The expression of B55α, Caveolin 1 and Sirtuin-1 is reduced in arteries from CLI mice and PAD patient, and the expression of these markers was restored in mice treated with VCE-004.8.
Conclusions: The findings presented in this study indicate that Etrinabdione holds promise in mitigating endothelial cell damage and senescence, while concurrently fostering arteriogenesis and angiogenesis. These observations position Etrinabdione as a compelling candidate for the treatment of PAD, and potentially other cardiovascular disorders.
Keywords: Angiogenesis; Etrinabdione; HIF-1α; PPA2/B55α; Sirtuin 1; VCE-004.8; eNOS.
© 2024. The Author(s).
Conflict of interest statement
AGM, ILC, and EM have applied for a European Patent based on some of the data presented herein.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

