Differentiating HER2-low and HER2-zero tumors with 21-gene multigene assay in 2,295 h + HER2- breast cancer: a retrospective analysis
- PMID: 39506855
- PMCID: PMC11542199
- DOI: 10.1186/s13058-024-01911-9
Differentiating HER2-low and HER2-zero tumors with 21-gene multigene assay in 2,295 h + HER2- breast cancer: a retrospective analysis
Erratum in
-
Correction: Differentiating HER2-low and HER2-zero tumors with 21-gene multigene assay in 2,295 HR + HER2- breast cancer: a retrospective analysis.Breast Cancer Res. 2024 Nov 26;26(1):164. doi: 10.1186/s13058-024-01929-z. Breast Cancer Res. 2024. PMID: 39593071 Free PMC article. No abstract available.
Abstract
Background: HER2-positivity is an essential marker for therapeutic decisions, while HER2 expression is heterogenous. In recent years, there has been increasing recognition of a subgroup of breast cancer patients who have low levels of HER2 expression, also known as HER2-low because trastuzumab deruxtecan offers clinical benefit for patients with HER2-low metastatic breast cancer. Despite the growing interest in HER2-low breast cancer, there is limited research on how multigene assays can help differentiate between HER2-low and HER2-negative breast cancer. Among HR + HER2- breast cancer, we compared genomic characteristics between HER2-low and HER2-zero using the 21-gene assay.
Methods: A retrospective review of clinical records was performed in 2,295 patients who underwent Oncotype DX® test in two hospitals between 2013 and 2020. Patients were classified into two groups as the HER2-zero and HER2-low based on HER2 immunohistochemistry. In cases with HER2 2+, no amplification of HER2 gene was confirmed by silver in situ hybridization. High genomic risk was defined as cases with 21-gene recurrence score (RS) > 25. Multivariable binary logistic-regression analysis was performed.
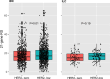
Results: Of these, 944 (41.1%) patients were assigned to the HER2-zero group, while 1351 (58.9%) patients were assigned to the HER2-low group. The average Recurrence Score (RS) was found to be 17.802 in the HER2-zero breast cancer group and 18.503 in the HER2-low group, respectively (p-value < 0.005). When comparing the proportion of high RS between the two groups, the HER2-zero group had a high RS rate of 12.4% (117 out of 944), while the HER2-low group had a high RS rate of 17.0% (230 out of 1351) (p = 0.002). The HER2 score identified by qRT-PCR was 8.912 in the HER2-zero group and 9.337 in the HER2-low group (p < 0.005). In multivariable analysis, HER2-low status was found to be an independent factor for high RS, with an odds ratio of 1.517 (1.172-1.964), independent of ER, PR, and Ki67. Within the subgroup of patients with invasive ductal carcinoma, the high RS rates were 19% in the HER2-low group and 14% in the HER2-zero group. However, when considering all patients, there were no significant differences observed in recurrence-free survival and overall survival between the HER2-low and HER2-zero groups.
Conclusion: Within HR + HER2- breast cancer, HER2-low tumors are associated with high RS, especially for histologically invasive ductal carcinoma. A prognostic influence of HER2-low expression among HR + HER2- breast cancer remains as an area that requires further study.
Keywords: 21-gene multigene assay; Breast cancer; HER2-low.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences 2001, 98(19):10869–10874. - PMC - PubMed
-
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N Engl J Med. 2004;351(27):2817–26. - PubMed
-
- Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga J-Y, Brain E, Causeret S, DeLorenzi M, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375(8):717–29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

