Single intravenous administration of oncolytic adenovirus TILT-123 results in systemic tumor transduction and immune response in patients with advanced solid tumors
- PMID: 39506856
- PMCID: PMC11539705
- DOI: 10.1186/s13046-024-03219-0
Single intravenous administration of oncolytic adenovirus TILT-123 results in systemic tumor transduction and immune response in patients with advanced solid tumors
Abstract
Background: A limitation of approved oncolytic viruses is their requirement for intratumoral (i.t.) injection. TILT-123 (igrelimogene litadenorepvec, Ad5/3-E2F-D24-hTNFα-IRES-hIL-2) is a chimeric oncolytic adenovirus suitable for intravenous (i.v.) delivery due to its capsid modification and dual selectivity devices. It is armed with tumor necrosis alpha and interleukin-2 for promoting T-cell activation and lymphocyte trafficking to tumors, thereby enhancing the antitumor immune response. Here, we present the findings after a single i.v. administration of TILT-123 in three phase I dose escalation clinical trials.
Methods: Patients with advanced solid tumors initially received a single i.v. dose of TILT-123 ranging from 3 × 109 to 4 × 1012 viral particles (VP). Blood was collected at baseline, 1, 16, and 192 h (7 days) post-treatment for bioavailability and serum analysis. Tumor biopsies were collected prior to treatment and 7 days post-treatment for analysis of viral presence and immunological effects. Patients did not receive any other cancer therapies during this period.
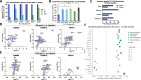
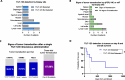
Results: Across all three trials (TUNIMO, TUNINTIL, and PROTA), 52 total patients were treated with i.v. TILT-123. Overall, TILT-123 was found to be well-tolerated, with no dose-limiting toxicities observed. Post-treatment tumor biopsies showed expression of viral genes, presence of TILT-123 adenovirus proteins or DNA, and changes in immune cell infiltration from baseline. Increased virus dose did not lead to increased virus detection in tumors. Median overall survival was longer in patients with confirmed presence of TILT-123 in post-treatment biopsies (280 versus 190 days, p = 0.0405).
Conclusion: TILT-123 demonstrated safety and significant intratumoral immunomodulation following a single i.v. administration, warranting further investigation.
Trial registrations: TUNIMO-NCT04695327. Registered 4 January 2021, https://clinicaltrials.gov/study/NCT04695327 . TUNINTIL-NCT04217473. Registered 19 December 2019, https://clinicaltrials.gov/study/NCT04217473 . PROTA-NCT05271318. Registered 4 February 2022, https://clinicaltrials.gov/study/NCT05271318 .
Keywords: Adenovirus; Immunotherapy; Intravenous Delivery; Oncolytic Virus; Solid Tumors; TILT-123.
© 2024. The Author(s).
Conflict of interest statement
AH is shareholder in Circio Holdings ASA. AH, VCC, JHAC, JMS, CK, are employees and shareholders of TILT Biotherapeutics Ltd. OH is a shareholder of TILT Biotherapeutics. LH, DCAQ, SS are employees of TILT Biotherapeutics. MSB and IMS receive institutional research support from TILT Biotherapeutics. MSB serves as an advisory board member for TILT Biotherapeutics. Other authors declare no conflicts of interest.
Figures



References
-
- Ovarian Cancer — Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed 14 Oct 2024.
-
- Bray F, Laversanne M, Hyuna, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. 10.3322/CAAC.21834. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

