The sodium/ascorbic acid co-transporter SVCT2 distributes in a striated membrane-enriched domain at the M-band level in slow-twitch skeletal muscle fibers
- PMID: 39506870
- PMCID: PMC11542426
- DOI: 10.1186/s40659-024-00554-6
The sodium/ascorbic acid co-transporter SVCT2 distributes in a striated membrane-enriched domain at the M-band level in slow-twitch skeletal muscle fibers
Abstract
Background: Vitamin C plays key roles in cellular homeostasis, functioning as a potent antioxidant and a positive regulator of cell differentiation. In skeletal muscle, the vitamin C/sodium co-transporter SVCT2 is preferentially expressed in oxidative slow fibers. SVCT2 is up-regulated during the early fusion of primary myoblasts and decreases during initial myotube growth, indicating the relevance of vitamin C uptake via SVCT2 for early skeletal muscle differentiation and fiber-type definition. However, our understanding of SVCT2 expression and function in adult skeletal muscles is still limited.
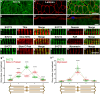
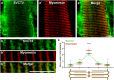
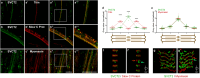
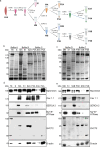
Results: In this study, we demonstrate that SVCT2 exhibits an intracellular distribution in chicken slow skeletal muscles, following a highly organized striated pattern. A similar distribution was observed in human muscle samples, chicken cultured myotubes, and isolated mouse myofibers. Immunohistochemical analyses, combined with biochemical cell fractionation experiments, reveal a strong co-localization of SVCT2 with intracellular detergent-soluble membrane fractions at the central sarcomeric M-band, where it co-solubilizes with sarcoplasmic reticulum proteins. Remarkably, electrical stimulation of cultured myofibers induces the redistribution of SVCT2 into a vesicular pattern.
Conclusions: Our results provide novel insights into the dynamic roles of SVCT2 in different intracellular compartments in response to functional demands.
Keywords: M-band; SVCT2; Sarcoplasmic reticulum; Skeletal muscle; Vitamin C.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

