Embedding patient engagement in the R&D process of a life sciences company through co-creation with a patient expert R&D board: a case study
- PMID: 39506875
- PMCID: PMC11539748
- DOI: 10.1186/s40900-024-00631-w
Embedding patient engagement in the R&D process of a life sciences company through co-creation with a patient expert R&D board: a case study
Abstract
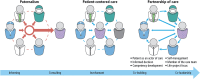
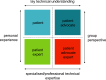
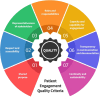
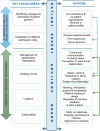
Patient involvement is crucial in healthcare, a factor increasingly recognised by life sciences companies and research institutes. This article presents a case study on Servier, a life sciences company that founded a patient expert board, ahead of launching a new research and development (R&D) institute. The aim was to foster a patient-centric culture within the company. The case study explores key developments in patient and public involvement, emphasising a shift from paternalistic to patient-centred approaches, noting few available case studies on patient board collaborations in life sciences. It outlines the evolution of the board, its impact, and practical lessons learned, with related recommendations. The patient board resulted from a three-way collaboration between the company, Patvocates (a patient consultancy), and patient experts recruited. The patient consultancy played a crucial role in project management, governance, and facilitating relationships. The case study provides the context, timeframe, foundations laid, engagement of patient experts, and foundational values, including: co-creation, fair market value remuneration, voluntary participation, and patient-centric meeting protocol. Eighteen patient experts, representing ten disease areas and ten European countries, joined the board and helped prioritise and co-create projects. Ideas for activities were sourced from brainstorming sessions and an in-company challenge. The collaboration yielded five core ideas, each forming a working group. The study describes the groups and their outputs: a patient advisory council, an interactive gallery of patient experience in R&D, patient engagement and entrepreneurship in life sciences, creating patient-focused decentralised trials (DCTs), and staff training on patient engagement. The article emphasises how the organic evolution of the collaboration led to significant insights. Hurdles faced by the company included: upstream planning, cross-company buy-in, compliance, and internal resource allocation. Recommendations for the wider community included: identifying and contracting patient partners; clarifying roles; managing expectations; building trust; logistics; and sustainability. This case study presents a practical, positive example of patient engagement within a life sciences company, offering insights into the establishing, running, and the impact of collaborating with a patient expert board. Lessons learned and recommendations may serve as a model for other companies seeking to engage with patients and evolve towards a more patient-centric approach in their strategies.
© 2024. The Author(s).
Conflict of interest statement
Some authors (SA, AL, LR, and GM) are full-time employees of Servier. No authors own Servier stocks. One author (JG) is CEO of the patient consultancy, Patvocates. One author (DS) is a part-time employee of Patvocates. One author (AA) is both a full-time employee of Patvocates and a member of the patient board. The co-corresponding author (EJ), and patient contributors (AA, SG, BN, TS, OS, LS, JW) are members of the Servier Saclay R&D Patient Expert Board, as described in the article, and their affiliation appears accordingly.
Figures
References
-
- World Health Organization. Constitution of the World Health Organization [Internet]. WHO; 1948 [cited 2024 Feb 1]. Available from: https://www.who.int/about/accountability/governance/constitution
-
- U.S. FDA PEAC. Patient Engagement in Medical Device Clinical Trials: Discussion Document [Internet]. U.S. FDA; 2018 [cited 2024 Feb 15]. Available from: https://www.fda.gov/media/117890/download
-
- U.S. FDA. Patient Engagement in the Design and Conduct of Medical Device Clinical Studies: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders [Internet]. Patient Engagem. Des. Conduct Med. Device Clin. Stud. FDA; 2022 [cited 2024 Feb 15]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
Publication types
LinkOut - more resources
Full Text Sources

