Exploring immunotherapy efficacy in non-small cell lung cancer patients with BRAF mutations: a case series and literature review
- PMID: 39507034
- PMCID: PMC11535819
- DOI: 10.21037/tlcr-24-253
Exploring immunotherapy efficacy in non-small cell lung cancer patients with BRAF mutations: a case series and literature review
Abstract
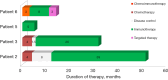
Background: The use of immunotherapy in treatment of non-small cell lung cancer (NSCLC) patients with the BRAF gene mutations is an area of active research and is an item of clinical trials. While BRAF mutations are relatively infrequent in NSCLC patients, comprising approximately 1-3% of cases, the V600E substitution stands out as the most prevalent subtype of BRAF mutations. The presence of this mutation in cancer cells qualifies the patients for first-line therapy with BRAF and MEK inhibitors. This study aims to evaluate the efficacy of immunotherapy in NSCLC patients with BRAF mutations. We presented a series of seven NSCLC cases with BRAF mutations, four of whom received immunotherapy or chemoimmunotherapy.
Methods: We observed benefit from immunotherapy in all patients, but its duration depended on comorbidities and the presence of brain metastases. Utilization of the next generation sequencing (NGS) technique causes high detection frequency of BRAF mutations (4.7% of patients), although mutations other than V600E may predominate (4 out of 7 patients).
Results: In patients receiving immune checkpoint inhibitors (ICIs)-based therapy, the median progression-free survival (PFS) was 17 months from the start of immunotherapy, the overall objective response rate (ORR) was 50%, and disease control was achieved in all patients.
Conclusions: Immunotherapy can benefit NSCLC patients with BRAF mutations, though its efficacy is affected by comorbidities and brain metastases. The use of NGS enhances mutation detection, highlighting the need for personalized treatment approaches in NSCLC management. The varying responses to treatments among the patients emphasize the complexity of NSCLC management and the necessity for a personalized approach.
Keywords: BRAF mutation; Non-small cell lung cancer (NSCLC); case series; immunotherapy.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-253/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials