Prognostic significance of bone metastasis and clinical value of bone radiotherapy in metastatic non-small cell lung cancer receiving PD-1/PD-L1 inhibitors: results from a multicenter, prospective, observational study
- PMID: 39507037
- PMCID: PMC11535830
- DOI: 10.21037/tlcr-24-441
Prognostic significance of bone metastasis and clinical value of bone radiotherapy in metastatic non-small cell lung cancer receiving PD-1/PD-L1 inhibitors: results from a multicenter, prospective, observational study
Abstract
Background: Bone metastasis (BoM) is a prevalent occurrence in patients with non-small cell lung cancer (NSCLC), significantly impacting prognosis and diminishing both survival rates and patients' quality of life. More and more studies have demonstrated that immunotherapy can improve the prognosis of NSCLC patients with bone metastases. Previous investigations pertaining to BoM in NSCLC have generally suffered from small sample sizes, absence of propensity score matching (PSM) to equate baseline characteristics, and an omission of the examination of patterns of treatment failure. This study aims to evaluate the prognostic significance of BoM and potential clinical value of bone radiation in metastatic NSCLC patients receiving immunotherapy.
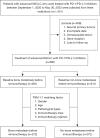
Methods: Metastatic NSCLC patients receiving programmed cell death protein 1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitors from three academic centers were enrolled in a prospective, observational trial (https://clinicaltrials.gov/study/NCT04766515) and those with measurable disease and adequate follow-up were retrospectively reviewed. Propensity score matched (PSM) patients with and without BoM were included in this study. Treatment efficacy, pattern of failure and clinical value of bone radiotherapy were extensively evaluated.
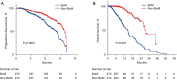
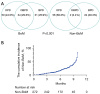
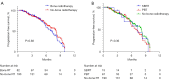
Results: A total of 544 out of 1,451 immunotherapy-treated NSCLC patients were included after PSM, including 272 with BoM and 272 without. Patients with baseline BoM had a median progression-free survival (PFS) of 7.8 months [95% confidence interval (CI): 7.0-8.7], lower than those without it (9.5 months; 95% CI: 8.9-10.0) (P<0.001). Patients with baseline BoM had a median overall survival (OS) of 14.5 months (95% CI: 12.6-16.4), lower than those without 27.6 months (95% CI: 25.1-30.1) (P<0.001). Patients with BoM also had lower objective response rate than those without it (11.1% vs. 15.8%, P<0.001). Initial disease progression in the bone was more common in those with BoM (56.5%) compared to those without it (31.7%) (P<0.001). Meanwhile, among patients with BoM, no significant difference of PFS was found between those receiving bone radiation or not, possibly due to a dominant use of palliative radiotherapy.
Conclusions: Baseline BoM correlated with worse prognosis and palliative bone radiation did not improve PFS in metastatic NSCLC patients receiving PD-1/PD-L1 inhibitors.
Keywords: Non-small cell lung cancer (NSCLC); bone metastasis (BoM); bone radiotherapy; programmed cell death protein 1/programmed cell death-ligand 1 inhibitors (PD-1/PD-L1 inhibitors); progression-free survival (PFS).
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-441/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
-
- Brahmer JR, Rodríguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol 2017;18:1600-9. 10.1016/S1470-2045(17)30690-3 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
