Burden of infant group B Streptococcus disease and impact of maternal screening and antibiotic prophylaxis in Ontario, Canada: a population-based cohort study
- PMID: 39507368
- PMCID: PMC11539648
- DOI: 10.1016/j.lana.2024.100914
Burden of infant group B Streptococcus disease and impact of maternal screening and antibiotic prophylaxis in Ontario, Canada: a population-based cohort study
Abstract
Background: Group B Streptococcus (GBS) significantly contributes to neonatal sepsis and meningitis, with varying disease rates reported globally and limited population-based data. We estimated infant GBS disease burden in Ontario, Canada and assessed the association of maternal GBS screening (35-37 weeks' gestation) and intrapartum antibiotic prophylaxis (IAP) provision with infant disease rates.
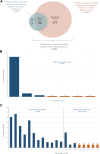
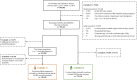
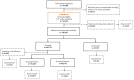
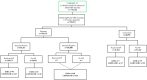
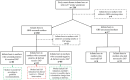
Methods: Our population-based cohort study included pregnant individuals and their offspring from April 2012 to March 2018, utilising the provincial birth registry linked to health administrative data. GBS cases were ascertained through culture results and diagnostic codes. We calculated incidence rates for early-onset disease (EOD: 0-6 days), late-onset disease (LOD: 7-89 days), and ultra-LOD (ULOD: 90-365 days). Adjusted incidence rate ratios (aIRR) were derived via log-binomial regression to compare infant GBS rates according to screening and IAP-receipt.
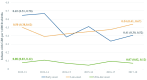
Findings: Among 776,148 liveborn infants, we identified 803 with GBS, with multiples exhibiting a threefold incidence increase. Incidence rates of EOD, LOD and ULOD were 0.49, 0.46 and 0.07 per 1000 livebirths, respectively. Of eligible pregnancies, 94% were screened; 23% screened positive, and 81% of them received IAP. Nearly 12% of term EOD infants had mothers who missed IAP despite screening positive. Maternal screening was associated with lower rates of any infant GBS disease (aIRR: 0.60; 95% CI: 0.45, 0.80). Among screen-positive births, IAP-receipt was associated with reduced rates of EOD (aIRR: 0.72, 95% CI: 0.48, 1.29) and LOD/ULOD (aIRR: 0.69; 95% CI: 0.46, 1.05), but confidence intervals included 1.0.
Interpretation: Our study, the largest Canadian investigation into infant GBS disease, highlights both widespread adoption and ongoing challenges of the current prevention strategy.
Funding: Canadian Institutes of Health Research.
Keywords: Burden; Early onset; Estimate; Group B Streptococcus; Incidence; Late onset; Streptococcus agalactiae.
© 2024 The Author(s).
Conflict of interest statement
MS has been an investigator on projects funded by Pfizer, Merck, Moderna, Sanofi Pasteur, and GlaxoSmithKline (all unrelated to study). All funds have been paid to his institute, and he has not received any personal payments. KW is the CEO of CANImmunize Inc and served as a member of the independent data safety monitoring committee for the Medicago COVD-19 vaccine trial. When this project was funded and initiated, DBF was employed by the University of Ottawa and had an academic appointment at the Children’s Hospital of Eastern Ontario Research Institute; she is now employed by Pfizer and works on an unrelated topic. DEC has been an investigator on projects funded by Moderna and Pfizer. All funds have been paid to her institute, and she has not received any personal payments. AM has received research funds to her institution from Pfizer and Sanofi Pasteur and received personal fees for advisory boards and DSMBs from Astra-Zeneca, GlaxoSmithKline, Medicago, Merck, Moderna, Pfizer, Roche and Sanofi Pasteur. SB is the director of the Centre for Vaccine Preventable Diseases at the University of Toronto, which has received support from Merck, Sanofi and Pfizer. All funds are paid to the institute and are subject to the University of Toronto’s governance policies.
Figures

References
-
- Costa N.S., Oliveira L.M.A., Meštrović T., Obiero C.W., Lee S.S., Pinto T.C.A. The urgent need to recognize and properly address prenatal-onset group B Streptococcus disease. Int J Infect Dis. 2022;124:168–170. - PubMed
-
- Money D., Allen V.M., Yudin M.H., et al. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Canada. 2013;35(10):939–948. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

