Targetable molecular algorithm and training platform development for the treatment of non-small cell lung cancer
- PMID: 39507406
- PMCID: PMC11540162
- DOI: 10.1093/jamiaopen/ooae124
Targetable molecular algorithm and training platform development for the treatment of non-small cell lung cancer
Abstract
Background and introduction: Over the last decade, treatment of patients with advanced non-small cell lung cancer (NSCLC) has become dependent on tissue and/or blood biomarkers to guide treatment decisions. Timely access to comprehensive biomarker and tumor signature information is crucial for diagnostic testing. With the rapid development and implementation of complex biomarker testing, comprehensive molecular profiling with in-depth analysis of DNA, RNA, and proteins can be easily performed. Initial data from the MedStar Health system showed considerable disparities in the use of next generation sequencing (NGS) between hospitals, and there is a clear need to improve education and understanding regarding which cases are appropriate for NGS, as well as the use of protocols to make those decisions in an expeditious manner.
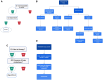
Materials and methods: Clinical pathways are systems-based tools that aim to create greater transparency around care decision making, therapeutic selection, and care delivery. They enhance quality and efficiency by reducing non-value-added intra-provider variability in care. We aimed to create a comprehensive clinical pathway system, the Targetable Molecular Algorithm (TMA), to increase the understanding and use of NGS in NSCLC by physicians in training, oncology nurse navigators, nurse practitioners, and general oncologists.
Discussion: We provide an overview of the implementation of the platform along with navigation guide. A realistic case study-a typical clinical workflow for a patient requiring NGS testing with an EGFR mutation-is also reviewed, demonstrating how the TMA platform can be applied. Additionally, we highlight the importance of the resource, and discuss its strengths, weaknesses, and potential future applications.
Results and conclusion: This new and innovative pathway system will make decision-making easier for clinicians trying to understand the appropriate tests and treatment algorithms for their patients. Our aim is to increase the appropriate and timely use of NGS among health-system providers with the hope that this system will empower physicians to provide better care by providing a quick, simple, user-friendly tool for comprehensive patient care.
Keywords: NSCLC; biomarkers; clinical pathways; electronic learning system; next generation sequencing; precision oncology; targetable molecular algorithm.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
J.R. is Advisory Board/Consultant at Genentech/Roche, Sanofi/Genzyme, Personalis, Guardant, Astrazeneca, BMS, Arcus, Abbvie, Daiichi Sankyo, Catalym. He receives research funding (to institution) from: Genentech/Roche, Verastem, Nuvalent, Mesothelioma Applied Research Foundation, LUNGevity Foundation. He has received honoraria/Speaking Fees from Astrazeneca, Merck. C.K. is Consultant/advisory board member at Novartis, Janssen, AstraZeneca, Sanofi, PierianDx, Diffuse pharmaceuticals, Mirati, Jazz Pharmaceuticals, Arcus Biosciences, Daiichi Sankyo, Eisai, Regeneron. He receives research funding (to institution) from: AstraZeneca, Bristol-Myers Squibb, Novartis, Genentech, Janssen, Regeneron, Debiopharm, Karyopharm, Daiichi-Sankyo, Lyell Immunopharma.
Figures


References
-
- Garraway LA, Verweij J, Ballman KV.. Precision oncology: an overview. JCO. 2013;31:1803-1805. - PubMed
-
- Gutierrez ME, Choi K, Lanman RB, et al.Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18:651-659. - PubMed
-
- MacLean E, Louder A, Saverno K, et al.Molecular testing patterns in metastatic non-small cell lung cancer. Am J Manag Care. 2016;22:e60-7. - PubMed
-
- Celine Audibert MS, Glass D, Kozak M, et al.Trends in the molecular diagnosis of lung cancer, results from an online market research survey. Friends Cancer Res. 2017.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
