Clinical significance of surgical resection for hepatocellular carcinoma with portal vein invasion: a nationwide cohort study
- PMID: 39507744
- PMCID: PMC11534781
- DOI: 10.21037/hbsn-23-578
Clinical significance of surgical resection for hepatocellular carcinoma with portal vein invasion: a nationwide cohort study
Abstract
Background: Hepatocellular carcinoma (HCC) with portal vein invasion (PVI) is considered an advanced stage with a poor prognosis. Although current guidelines recommend systemic treatment for HCC with PVI, surgical resection could produce acceptable outcomes in selected patients. This study aimed to identify the clinical significance of surgical resection for HCC with PVI patients using a large-scale nationwide registry.
Methods: This retrospective, multicenter, observational cohort analyzed data from the Korean Primary Liver Cancer Registry. A total of 16,781 patients who were newly diagnosed with HCC between 2008 and 2018 were enrolled in this study. Patients with worse Child-Turcotte-Pugh scores (≥7) or performance status (≥2) were excluded. Among them, 998 patients who received treatment for HCC with PVI were included in the analysis and were divided into two groups: resection group of 151 (15.1%) and palliative group of 847 (84.9%) who received transarterial and systemic therapy according to the treatment intent. After matching the number and size of the tumors and model for end-stage liver disease (MELD) score between the groups, the final study cohort for analysis comprised 151 (26.6%) patients in the resection group and 417 (73.4%) in the palliative group. The primary endpoints were overall survival (OS) and cancer-specific survival (CSS).
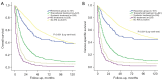
Results: The number and maximum size of HCC did not differ between the resection and palliative groups after matching [1 (range, 1-5) vs. 1 (range, 1-6), P=0.11 and 5.5 (range, 1.2-20.6) vs. 6.0 (range, 1.0-20.5) cm, P=0.24, respectively]. Tumor markers, including alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II), also did not differ between the groups (P=0.29 and P=0.36, respectively). The 5-year OS and CSS rates of the resection and palliative groups were 44.8% and 17.4% (P<0.001) and 47.7% and 18.6% (P<0.001), respectively. Multivariate analysis showed that palliative treatment intent was the most significant risk factor for OS and CSS [odds ratio (OR) =2.24; 95% confidence interval (CI): 1.66-3.02; P<0.001 and OR =2.29; 95% CI: 1.68-3.12; P<0.001, respectively].
Conclusions: Surgical resection could significantly improve OS and CSS in selected HCC with PVI patients who have preserved liver function and performance status.
Keywords: Hepatocellular carcinoma (HCC); cancer-specific survival (CSS); overall survival (OS); portal vein invasion (PVI); surgical resection.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-578/coif). The authors have no conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
