Non-local impact of distal airway constrictions on patterns of inhaled particle deposition
- PMID: 39508002
- PMCID: PMC11539137
- DOI: 10.1098/rsos.241108
Non-local impact of distal airway constrictions on patterns of inhaled particle deposition
Abstract
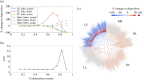
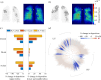
Airway constriction and blockage in obstructive lung diseases cause ventilation heterogeneity and create barriers to effective drug deposition. Established computational particle-deposition models have not accounted for these impacts of disease. We present a new particle-deposition model that calculates ventilation based on the resistance of each airway, such that ventilation responds to airway constriction. The model incorporates distal airway constrictions representative of cystic fibrosis, allowing us to investigate the resulting impact on patterns of deposition. Unlike previous models, our model predicts how constrictions affect deposition in airways throughout the lungs, not just in the constricted airways. Deposition is reduced in airways directly distal and proximal to constrictions. When constrictions are clustered together, central-airways deposition can increase significantly in regions away from constrictions, but distal-airways deposition in those regions remains largely unchanged. We use our model to calculate lung clearance index (LCI), a clinical measure of ventilation heterogeneity, after applying constrictions of varying severities in one lobe. We find an increase in LCI coinciding with significantly reduced deposition in the affected lobe. Our results show how the model provides a framework for development of computational tools that capture the impacts of airway disease, which could significantly affect predictions of regional dosing.
Keywords: airway disease; cystic fibrosis; inhaled therapies; network modelling; particle deposition.
© 2024 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
