Stem cell-derived exosomes for ischemic stroke: a conventional and network meta-analysis based on animal models
- PMID: 39508049
- PMCID: PMC11537945
- DOI: 10.3389/fphar.2024.1481617
Stem cell-derived exosomes for ischemic stroke: a conventional and network meta-analysis based on animal models
Abstract
Objective: We aimed to evaluate the efficacy of stem cell-derived exosomes for treating ischemic stroke and to screen for the optimal administration strategy.
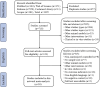
Methods: We searched PubMed, Web of Science, Embase, Cochrane Library, and Scopus databases for relevant studies published from their inception to 31 December 2023. Conventional and network meta-analyses of the routes of administration, types, and immune compatibility of stem cell-derived exosomes were performed using the cerebral infarct volume (%) and modified neurological severity score (mNSS) as outcome indicators.
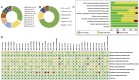
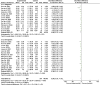
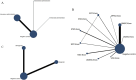
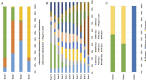
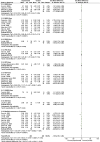
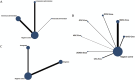
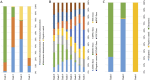
Results: A total of 38 randomized controlled animal experiments were included. Conventional meta-analysis showed that compared with the negative control group: intravenous administration significantly reduced the cerebral infarct volume (%) and mNSS; intranasal administration significantly reduced the cerebral infarct volume (%); and intracerebral administration significantly reduced the mNSS. Adipose-derived mesenchymal stem cell-derived exosomes (ADSC-Exos), bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos), dental pulp stem cell-derived exosomes (DPSC-Exos) and neural stem cell-derived exosomes (NSC-Exos) significantly reduced the cerebral infarct volume (%) and mNSS; Endothelial progenitor cell-derived exosomes (EPC-Exos), embryonic stem cell-derived exosomes (ESC-Exos), induced pluripotent stem cell-derived exosomes (iPSC-Exos) and neural progenitor cell-derived exosomes (NPC-Exos) significantly reduced the cerebral infarct volume (%); Umbilical cord mesenchymal stem cell-derived exosomes (UCMSC-Exos) significantly reduced the mNSS; and there was no significant difference between urogenital stem cell-derived exosomes (USC-Exos) and negative controls. Engineered modified exosomes had better efficacy than unmodified exosomes. Both allogeneic and xenogeneic stem cell-derived exosomes significantly reduced the cerebral infarct volume (%) and the mNSS. The network meta-analysis showed that intravenous administration was the best route of administration for reducing the cerebral infarct volume (%) and mNSS. Among the 10 types of stem cell-derived exosomes that were administered intravenously, BMSC-Exos were the best type for reducing the cerebral infarct volume (%) and the mNSS. Allogeneic exosomes had the best efficacy in reducing the cerebral infarct volume (%), whereas xenogeneic stem cell-derived exosomes had the best efficacy in reducing the mNSS.
Conclusion: This meta-analysis, by integrating the available evidence, revealed that intravenous administration is the best route of administration, that BMSC-Exos are the best exosome type, that allogeneic exosomes have the best efficacy in reducing the cerebral infarct volume (%), and that xenogeneic exosomes have the best efficacy in reducing mNSS, which can provide options for preclinical studies. In the future, more high-quality randomized controlled animal experiments, especially direct comparative evidence, are needed to determine the optimal administration strategy for stem cell-derived exosomes for ischemic stroke.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42024497333, PROSPERO, CRD42024497333.
Keywords: animal experiments; animal models; exosomes; meta-analysis; stem cells; stroke.
Copyright © 2024 Xu, Zhao, He, Guo and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes.Exp Neurol. 2021 Jul;341:113700. doi: 10.1016/j.expneurol.2021.113700. Epub 2021 Mar 17. Exp Neurol. 2021. PMID: 33741350
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p.Biomolecules. 2022 Jun 24;12(7):883. doi: 10.3390/biom12070883. Biomolecules. 2022. PMID: 35883438 Free PMC article.
-
Bone marrow mesenchymal stem cell-derived exosomal lncRNA KLF3-AS1 stabilizes Sirt1 protein to improve cerebral ischemia/reperfusion injury via miR-206/USP22 axis.Mol Med. 2023 Jan 10;29(1):3. doi: 10.1186/s10020-022-00595-1. Mol Med. 2023. PMID: 36627572 Free PMC article.
-
Stem cell-derived exosomes in the treatment of acute myocardial infarction in preclinical animal models: a meta-analysis of randomized controlled trials.Stem Cell Res Ther. 2022 Apr 8;13(1):151. doi: 10.1186/s13287-022-02833-z. Stem Cell Res Ther. 2022. PMID: 35395872 Free PMC article. Review.
Cited by
-
Advances in brain remodeling, stem cell therapies, and translational barriers in stroke and brain aging.Biogerontology. 2025 Jul 11;26(4):143. doi: 10.1007/s10522-025-10282-3. Biogerontology. 2025. PMID: 40643679 Free PMC article. Review.
-
Human induced neural progenitor cells generated from three-dimensional aggregate-based culture significantly improve post-stroke recovery in tMCAO mice.Stem Cell Res Ther. 2025 Jun 20;16(1):312. doi: 10.1186/s13287-025-04433-z. Stem Cell Res Ther. 2025. PMID: 40542444 Free PMC article.
-
Cerebral ischemia-reperfusion injury: mechanisms and promising therapies.Front Pharmacol. 2025 Jul 16;16:1613464. doi: 10.3389/fphar.2025.1613464. eCollection 2025. Front Pharmacol. 2025. PMID: 40766753 Free PMC article. Review.
-
Brain Endothelial Cells in Blood-Brain Barrier Regulation and Neurological Therapy.Int J Mol Sci. 2025 Jun 18;26(12):5843. doi: 10.3390/ijms26125843. Int J Mol Sci. 2025. PMID: 40565303 Free PMC article. Review.
-
Harnessing bacterial metabolites for enhanced cancer chemotherapy: unveiling unique therapeutic potentials.Arch Microbiol. 2024 Oct 29;206(11):449. doi: 10.1007/s00203-024-04179-x. Arch Microbiol. 2024. PMID: 39472338 Review.
References
-
- Campero-Romero A. N., Real F. H., Santana-Martínez R. A., Molina-Villa T., Aranda C., Ríos-Castro E., et al. (2023). Extracellular vesicles from neural progenitor cells promote functional recovery after stroke in mice with pharmacological inhibition of neurogenesis. Cell. Death Discov. 9, 272. 10.1038/s41420-023-01561-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

