A 5-year natural history cohort of patients with facioscapulohumeral muscular dystrophy determining disease progression and feasibility of clinical outcome assessments for clinical trials
- PMID: 39508285
- PMCID: PMC11632561
- DOI: 10.1002/mus.28293
A 5-year natural history cohort of patients with facioscapulohumeral muscular dystrophy determining disease progression and feasibility of clinical outcome assessments for clinical trials
Abstract
Introduction/aims: The number of clinical trials in facioscapulohumeral muscular dystrophy (FSHD) is expected to increase in the near future. There is a need for clinical outcome assessments (COAs) that can capture disease progression over the relatively short time span of a clinical trial. In this study, we report the natural progression of FSHD and determine the feasibility of COAs for clinical trials.
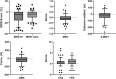
Methods: Genetically confirmed FSHD patients underwent various COAs at baseline and after 5 years. COAs consisted of the Motor Function Measure (MFM), manual muscle testing using the Medical Research Council score, six-minute walk test, quantitative muscle strength assessment of the quadriceps muscle, clinical severity score, and FSHD evaluation score (FES). Statistical significance and the minimal clinically important difference (MCID) were calculated and power calculations were performed.
Results: One hundred fifty-four symptomatic FSHD patients were included, with a mean (SD) age of 51.4 (14.6) years old. All COAs showed a minimal, yet statistically significant progression after 5 years. MCID was reached for the MFM Domain 1, MFM total score, and FES. These three COAs showed the lowest sample size requirements for clinical trials (185, 156, and 201 participants per group, respectively, for a trial duration of 2 years).
Discussion: The captured FSHD disease progression rate in 5 years was generally minimal. The COAs in this study are not feasible for clinical trials with a duration of 2 years. Extended trial durations or novel outcome assessments might be necessary to improve trial feasibility in FSHD.
Keywords: FSHD; clinical outcome assessments; disease progression; minimal clinically important difference; power calculation.
© 2024 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures

References
-
- Tawil R, Shaw DW, van der Maarel SM, Tapscott SJ. Clinical trial preparedness in facioscapulohumeral dystrophy: outcome measures and patient access: 8‐9 April 2013, Leiden, The Netherlands. Neuromuscu Disord. 2014;24(1):79‐85. - PubMed
-
- Efficacy and Safety of Losmapimod in Treating Participants With Facioscapulohumeral Muscular Dystrophy (FSHD) (REACH) . ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of medicine (US). 2000 Feb 29 – Identifier NCT05397470, facioscapulohumeral dystrophy (FSHD) [cited 2024 Feb 21]. https://clinicaltrials.gov/study/NCT05397470?cond=FSHD&rank=2
-
- A Study to Evaluate RO7204239 in Participants With Facioscapulohumeral Muscular Dystrophy (MANOEUVRE) . ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of medicine (US). 2000 Feb 29 – Identifier NCT05548556, facioscapulohumeral dystrophy (FSHD) [cited 2024 Feb 21]. https://clinicaltrials.gov/study/NCT05548556?cond=FSHD&page=3&rank=24.
-
- Phase 1/2 Study of AOC 1020 in Adults With Facioscapulohumeral Muscular Dystrophy (FSHD) (FORTITUDE) ClinicalTrials.gov [Internet] . Bethesda (MD): National Library of medicine (US). 2000 Feb 29 – Identifier NCT05747924, facioscapulohumeral dystrophy (FSHD); [cited 2024 Feb 21]. https://clinicaltrials.gov/study/NCT05747924?cond=FSHD&page=4&rank=31.
-
- Tawil R, Wagner KR, Hamel JI, et al. Safety and efficacy of losmapimod in facioscapulohumeral muscular dystrophy (ReDUX4): a randomised, double‐blind, placebo‐controlled phase 2b trial. Lancet Neurol. 2024;23(5):477‐486. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

