Inherited kidney disease and CAKUT are common causes of kidney failure requiring kidney replacement therapy: an ERA Registry study
- PMID: 39508350
- PMCID: PMC12035533
- DOI: 10.1093/ndt/gfae240
Inherited kidney disease and CAKUT are common causes of kidney failure requiring kidney replacement therapy: an ERA Registry study
Abstract
Background: Inherited kidney diseases (IKDs) and congenital anomalies of the kidney and urinary tract (CAKUT) are causes of kidney failure requiring kidney replacement therapy (KRT) that major renal registries usually amalgamate into the primary renal disease(PRD) category 'miscellaneous' or in the glomerulonephritis or pyelonephritis categories. This makes IKDs invisible (except for polycystic kidney disease) and may negatively influence the use of genetic testing, which may identify a cause for IKDs and some CAKUT.
Methods: We re-examined the aetiology of KRT by composing a separate IKD and CAKUT PRD group using data from the European Renal Association (ERA) Registry.
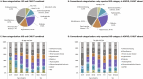
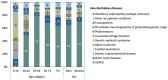
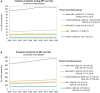
Results: In 2019, IKD-CAKUT was the fourth most common cause of kidney failure among incident KRT patients, accounting for 8.9% of cases [IKD 7.4% (including 5.0% autosomal dominant polycystic kidney disease), CAKUT 1.5%], behind diabetes (23.0%), hypertension (14.4%) and glomerulonephritis (10.6%). IKD-CAKUT was the most common cause of kidney failure among patients <20 years of age (41.0% of cases), but their incidence rate was highest among those ages 45-74 years (22.5 per million age-related population). Among prevalent KRT patients, IKD-CAKUT (18.5%) and glomerulonephritis (18.7%) were the two most common causes of kidney failure overall, while IKD-CAKUT was the most common cause in women (21.6%) and in patients <45 years of age (29.1%).
Conclusion: IKD and CAKUT are common causes of kidney failure among KRT patients. Distinct categorization of IKD and CAKUT better characterizes the epidemiology of the causes of chronic kidney disease (CKD) and highlights the importance of genetic testing in the diagnostic workup of CKD.
Keywords: CAKUT; aetiology; epidemiology; genetic kidney disease; inherited kidney disease; kidney failure; kidney replacement therapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
A.O. reports grants from Sanofi and consultancy/speaker fees or travel support from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Idorsia, Chiesi, Otsuka, Novo Nordisk and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra AstraZeneca-UAM of chronic kidney disease and electrolytes. G.A. reports lecture/speaker fees from Alexion Pharmaceuticals, Recordati Rare Disease, Advicenne, Chiesi, Kyowa Kirim and Alnylam; travel support from Recordati Rare Disease, Advicenne, Chiesi and Alnylam and board membership for Alexion Pharmaceuticals, Advicenne, Dicerna, Alnylam and Novo Nordisk. P.M.F. reports royalties or licenses from UpToDate; consulting/lecture fees from Allena Pharmaceuticals, Alnylam, AstraZeneca, Bayer, Gilead, Novo Nordisk and Otsuka Pharmaceuticals and board membership for Allena Pharmaceuticals, Alnylam, AstraZeneca and Novo Nordisk. S.M. reports board membership for the Scottish Renal Registry (Public Health Scotland). R.S. reports lecture/speaker fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Menarini and travel support from Menarini. H.R. reports membership in the Bantao Association Board, the MKS Instruments board, the European Association of Professors Emeriti Board. M.S. reports grants from Njurfonden, SUS stiftelser och fonder and ALF (Avtal om Läkarutbildning och Forskning); consulting fees from Hansa Biopharma, Otsuka and Vifor and board membership for the Swedish Renal Registry. P.A. reports board membership for the Swiss Renal Registry and Quality Assessment Program. L.P. reports research grants from the National Institute for Health and Care Research and Academy of Medical Sciences and Kidney Research UK and support from the UK Kidney Association in the role as Paediatric Research Lead for the UK Renal Registry and a seconded role as Paediatric Research Lead of the UK Renal Registry. R.P. reports grants from the Icelandic Research Fund (Rannís) 2021–2024; travel grants from the Nordic Fabry Expert Group and board membership for the Icelandic Society of Transplantation and the Icelandic Society of Internal Medicine. K.J.J. reports board membership for the SharE RR working group of the International Society of Nephrology, all outside the submitted work. R.T. reports consultancy/speaker fees or travel support from Amicus, Sanofi-Genzyme, Kyowa Kirin, Alexion, Chiesi, Otsuka, Recordatti, Takeda, Alnylam, AstraZeneca and GSK. The remaining authors declare that they have no conflicts of interest.
Figures



References
-
- ERA-EDTA Registry . ERA-EDTA Registry Annual Report 1998. Amsterdam: Department of Medical Informatics, 2003. https://www.era-online.org/research-education/era-registry (30 November 2022, date last accessed).
-
- United States Renal Data System . 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2022. https://usrds-adr.niddk.nih.gov/2022 (30 November 2022, date last accessed).
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

