Efficient enzyme-free isolation of brain-derived extracellular vesicles
- PMID: 39508423
- PMCID: PMC11541858
- DOI: 10.1002/jev2.70011
Efficient enzyme-free isolation of brain-derived extracellular vesicles
Abstract
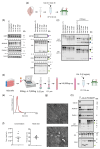
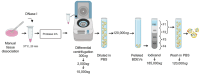
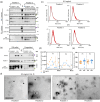
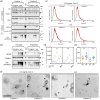
Extracellular vesicles (EVs) have gained significant attention as pathology mediators and potential diagnostic tools for neurodegenerative diseases. However, isolation of brain-derived EVs (BDEVs) from tissue remains challenging, often involving enzymatic digestion steps that may compromise the integrity of EV proteins and overall functionality. Here, we describe that collagenase digestion, commonly used for BDEV isolation, produces undesired protein cleavage of EV-associated proteins in brain tissue homogenates and cell-derived EVs. In order to avoid this effect, we studied the possibility of isolating BDEVs with a reduced amount of collagenase or without any protease. Characterization of the isolated BDEVs from mouse and human samples (both female and male) revealed their characteristic morphology and size distribution with both approaches. However, we show that even minor enzymatic digestion induces 'artificial' proteolytic processing in key BDEV markers, such as Flotillin-1, CD81, and the cellular prion protein (PrPC), whereas avoiding enzymatic treatment completely preserves their integrity. We found no major differences in mRNA and protein content between non-enzymatically and enzymatically isolated BDEVs, suggesting that the same BDEV populations are purified with both approaches. Intriguingly, the lack of Golgi marker GM130 signal, often referred to as contamination indicator (or negative marker) in EV preparations, seems to result from enzymatic digestion rather than from its actual absence in BDEV samples. Overall, we show that non-enzymatic isolation of EVs from brain tissue is possible and avoids artificial pruning of proteins while achieving an overall high BDEV yield and purity. This protocol will help to understand the functions of BDEV and their associated proteins in a near-physiological setting, thus opening new research approaches.
Keywords: BDEVs; EV corona; GM130; PrPC; brain‐derived EVs; collagenase‐free; prion protein; proteomics.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Antoniou, A. , Auderset, L. , Kaurani, L. , Sebastian, E. , Zeng, Y. , Allahham, M. , Cases‐Cunillera, S. , Schoch, S. , Gründemann, J. , Fischer, A. , & Schneider, A. (2023). Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Reports, 42, 112063. - PubMed
-
- Bachurski, D. , Schuldner, M. , Nguyen, P. H. , Malz, A. , Reiners, K. S. , Grenzi, P. C. , Babatz, F. , Schauss, A. C. , Hansen, H. P. , Hallek, M. , & Pogge von Strandmann, E. (2019). Extracellular vesicle measurements with nanoparticle tracking analysis—An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. Journal of Extracellular Vesicles, 8, 1596016. - PMC - PubMed
-
- Brenna, S. , Altmeppen, H. C. , Mohammadi, B. , Rissiek, B. , Schlink, F. , Ludewig, P. , Krisp, C. , Schlüter, H. , Failla, A. V. , Schneider, C. , Glatzel, M. , Puig, B. , & Magnus, T. (2020). Characterization of brain‐derived extracellular vesicles reveals changes in cellular origin after stroke and enrichment of the prion protein with a potential role in cellular uptake. Journal of Extracellular Vesicles, 9, 1809065. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 101030402_EXOSOMES AD/H2020 Marie Skłodowska-Curie Actions
- NWF-20/10/Forschungsförderungsfonds der Medizinischen Fakultät, University Medical Center Hamburg-Eppendorf
- INST337/15-1/Deutsche Forschungsgemeinschaft (DFG)
- INST337/16-1/Deutsche Forschungsgemeinschaft (DFG)
- INST152/837-1/Deutsche Forschungsgemeinschaft (DFG)
- INST152/947-1/Deutsche Forschungsgemeinschaft (DFG)
- PIF2020-10/PIER seed grant, University of Hamburg
- Creutzfeldt-Jakob Disease Foundation
- PHM-2019-03/PIER Hamburg/Boston seed grant, University of Hamburg
- Joachim Herz Stiftung
- Alzheimer Forschung Initiative
- 202108080249/Chinese Government Scholarship
LinkOut - more resources
Full Text Sources
Research Materials

