An updated review of HSV-1 infection-associated diseases and treatment, vaccine development, and vector therapy application
- PMID: 39508503
- PMCID: PMC11562918
- DOI: 10.1080/21505594.2024.2425744
An updated review of HSV-1 infection-associated diseases and treatment, vaccine development, and vector therapy application
Abstract
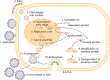
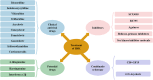
Herpes simplex virus type 1 (HSV-1) is a globally widespread virus that causes and associates with a wide range of diseases, including herpes simplex encephalitis, herpes simplex keratitis, and herpes labialis. The interaction between HSV-1 and the host involves complex immune response mechanisms, including recognition of viral invasion, maintenance of latent infection, and triggering of reactivation. Antiviral therapy is the core treatment for HSV-1 infections. Meanwhile, vaccine development employs different strategies and methods, and several promising vaccine types have emerged, such as live attenuated, protein subunit, and nucleic acid vaccines, offering new possibilities for the prevention of HSV-1 infection. Moreover, HSV-1 can be modified into a therapeutic vector for gene therapy and tumour immunotherapy. This review provides an in-depth summary of HSV-1 infection-associated innate and adaptive immune responses, disease pathogenesis, current therapeutic approaches, recent advances in vaccine development, and vector therapy applications for cancer treatment. Through a systematic review of multiple aspects of HSV-1, this study aims to provide a comprehensive and detailed reference for the public on the prevention, control, and treatment of HSV-1.
Keywords: HSV-1 vaccine; Herpes simplex virus 1; herpes labialis; herpes simplex keratitis; herpes simplex meningitis; immune response.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection.J Zhejiang Univ Sci B. 2017 Apr.;18(4):277-288. doi: 10.1631/jzus.B1600460. J Zhejiang Univ Sci B. 2017. PMID: 28378566 Free PMC article. Review.
-
Reduced immune responses after vaccination with a recombinant herpes simplex virus type 1 vector in the presence of antiviral immunity.J Gen Virol. 2005 Sep;86(Pt 9):2401-2410. doi: 10.1099/vir.0.81104-0. J Gen Virol. 2005. PMID: 16099897
-
Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses.Viruses. 2021 Aug 18;13(8):1637. doi: 10.3390/v13081637. Viruses. 2021. PMID: 34452501 Free PMC article. Review.
-
Herpes Simplex Virus 1 (HSV-1) 0ΔNLS Live-Attenuated Vaccine Protects against Ocular HSV-1 Infection in the Absence of Neutralizing Antibody in HSV-1 gB T Cell Receptor-Specific Transgenic Mice.J Virol. 2020 Nov 23;94(24):e01000-20. doi: 10.1128/JVI.01000-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999018 Free PMC article.
-
Impact of Type I Interferon on the Safety and Immunogenicity of an Experimental Live-Attenuated Herpes Simplex Virus 1 Vaccine in Mice.J Virol. 2017 Mar 13;91(7):e02342-16. doi: 10.1128/JVI.02342-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28122977 Free PMC article.
Cited by
-
Post-translational modifications as a key mechanism for herpes simplex virus type I evasion of host innate immunity.Front Microbiol. 2025 Feb 11;16:1543676. doi: 10.3389/fmicb.2025.1543676. eCollection 2025. Front Microbiol. 2025. PMID: 40008039 Free PMC article. Review.
-
The Procaine-Based ProcCluster® Impedes the Second Envelopment Process of Herpes Simplex Virus Type 1.Int J Mol Sci. 2025 Jul 25;26(15):7185. doi: 10.3390/ijms26157185. Int J Mol Sci. 2025. PMID: 40806319 Free PMC article.
-
Co-expression of HSV-1 ICP34.5 enhances the expression of gene delivered by self-amplifying RNA and mitigates its immunogenicity.FEBS Open Bio. 2025 Jul;15(7):1079-1089. doi: 10.1002/2211-5463.70036. Epub 2025 Apr 9. FEBS Open Bio. 2025. PMID: 40200732 Free PMC article.
-
The relationship between ferroptosis and respiratory infectious diseases: a novel landscape for therapeutic approach.Front Immunol. 2025 Mar 18;16:1550968. doi: 10.3389/fimmu.2025.1550968. eCollection 2025. Front Immunol. 2025. PMID: 40170865 Free PMC article. Review.
-
Oncoviruses in the Oral Cavity: Recent Advances in Understanding Viral Infections and Tumorigenesis.Int J Mol Sci. 2025 Jul 13;26(14):6721. doi: 10.3390/ijms26146721. Int J Mol Sci. 2025. PMID: 40724971 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
