Study of excess manganese stress response highlights the central role of manganese exporter Mnx for holding manganese homeostasis in the cyanobacterium Synechocystis sp. PCC 6803
- PMID: 39508727
- PMCID: PMC11649195
- DOI: 10.1099/mic.0.001515
Study of excess manganese stress response highlights the central role of manganese exporter Mnx for holding manganese homeostasis in the cyanobacterium Synechocystis sp. PCC 6803
Abstract
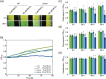
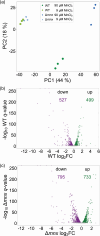
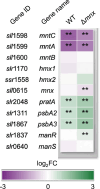
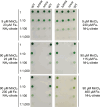
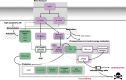
Cellular levels of the essential micronutrient manganese (Mn) need to be carefully balanced within narrow borders. In cyanobacteria, a sufficient Mn supply is critical for ensuring the function of the oxygen-evolving complex as the central part of the photosynthetic machinery. However, Mn accumulation is fatal for the cells. The reason for the observed cytotoxicity is unclear. To understand the causality behind Mn toxicity in cyanobacteria, we investigated the impact of excess Mn on physiology and global gene expression in the model organism Synechocystis sp. PCC 6803. We compared the response of the WT and the knock-out mutant in the Mn exporter (Mnx), ∆mnx, which is disabled in the export of surplus Mn and thus functions as a model for toxic Mn overaccumulation. While growth and pigment accumulation in ∆mnx were severely impaired 24 h after the addition of tenfold Mn, the WT was not affected and thus mounted an adequate transcriptional response. RNA-seq data analysis revealed that the Mn stress transcriptomes partly resembled an iron limitation transcriptome. However, the expression of iron limitation signature genes isiABDC was not affected by the Mn treatment, indicating that Mn excess is not accompanied by iron limitation in Synechocystis. We suggest that the ferric uptake regulator, Fur, gets partially mismetallated under Mn excess conditions and thus interferes with an iron-dependent transcriptional response. To encounter mismetallation and other Mn-dependent problems on a protein level, the cells invest in transcripts of ribosomes, proteases and chaperones. In the case of the ∆mnx mutant, the consequences of the disability to export excess Mn from the cytosol manifest in additionally impaired energy metabolism and oxidative stress transcriptomes with a fatal outcome. This study emphasizes the central importance of Mn homeostasis and the transporter Mnx's role in restoring and holding it.
Keywords: RNA-seq; cyanobacteria; manganese; regulation; toxicity; transporter.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The Synechocystis Manganese Exporter Mnx Is Essential for Manganese Homeostasis in Cyanobacteria.Plant Physiol. 2017 Mar;173(3):1798-1810. doi: 10.1104/pp.16.01895. Epub 2017 Jan 30. Plant Physiol. 2017. PMID: 28153926 Free PMC article.
-
The hierarchy of transition metal homeostasis: iron controls manganese accumulation in a unicellular cyanobacterium.Biochim Biophys Acta. 2014 Dec;1837(12):1990-1997. doi: 10.1016/j.bbabio.2014.09.007. Biochim Biophys Acta. 2014. PMID: 25261790
-
The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803.New Phytol. 2017 Jul;215(1):256-268. doi: 10.1111/nph.14526. Epub 2017 Mar 20. New Phytol. 2017. PMID: 28318016
-
Acclimation to environmentally relevant Mn concentrations rescues a cyanobacterium from the detrimental effects of iron limitation.Environ Microbiol. 2015 Jun;17(6):2090-8. doi: 10.1111/1462-2920.12826. Epub 2015 Mar 27. Environ Microbiol. 2015. PMID: 25728137
-
Manganese Homeostasis in Cyanobacteria.Plants (Basel). 2019 Dec 23;9(1):18. doi: 10.3390/plants9010018. Plants (Basel). 2019. PMID: 31877921 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

