Slc26a2-mediated sulfate metabolism is important in tooth development
- PMID: 39508796
- PMCID: PMC11655027
- DOI: 10.1242/dmm.052107
Slc26a2-mediated sulfate metabolism is important in tooth development
Abstract
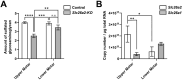
The sulfate transporter gene SLC26A2 is crucial for skeletal formation, as evidenced by its role in diastrophic dysplasia, a type of skeletal dysplasia in humans. Although SLC26A2-related chondrodysplasia also affects craniofacial and tooth development, its specific role in these processes remains unclear. In this study, we explored the pivotal roles of SLC26A2-mediated sulfate metabolism during tooth development. We found that Slc26a2 was predominantly expressed in dental tissues, including odontoblasts and ameloblasts. Slc26a2 knockout (Slc26a2-KO-Δexon2) mice exhibited distinct craniofacial abnormalities, such as a retrognathic upper jaw, small upper incisors and upper molar hypoplasia. These mice also showed flattened odontoblasts and loss of nuclear polarity in upper incisors and molars, with significant reductions in odontoblast differentiation markers Dspp and Dmp1. Ex vivo and in vitro studies further revealed dentin matrix hypoplasia, tooth root shortening and downregulation of Wnt signaling in Slc26a2-deficient cells. These findings highlight the crucial role of SLC26A2-mediated sulfate metabolism in tooth development and offer insights into the mechanisms underlying dental abnormalities in patients with SLC26A2-related chondrodysplasias.
Keywords: Extracellular matrix; Matrix biology; Odontoblasts; SLC26A2; Sulfate metabolism; Tooth development.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
-
- Bulfone, A., Kim, H. J., Puelles, L., Porteus, M. H., Grippo, J. F. and Rubenstein, J. L. R. (1993). The mouse Dlx-2 (Tes-1) gene is expressed in spatially restricted domains of the forebrain, face and limbs in midgestation mouse embryos. Mech. Dev. 40, 129-140. 10.1016/0925-4773(93)90071-5 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

