NT-proBNP improves prediction of cardiorenal complications in type 2 diabetes: the Hong Kong Diabetes Biobank
- PMID: 39508878
- PMCID: PMC11732878
- DOI: 10.1007/s00125-024-06299-x
NT-proBNP improves prediction of cardiorenal complications in type 2 diabetes: the Hong Kong Diabetes Biobank
Abstract
Aims/hypothesis: N-terminal pro B-type natriuretic peptide (NT-proBNP) is a natriuretic peptide that is strongly associated with congestive heart failure (CHF). The utility of NT-proBNP for prediction of cardiovascular events and renal endpoints, compared with clinical risk factors, has not been evaluated in detail. We hypothesise that NT-proBNP can improve risk stratification and prediction of cardiorenal events in type 2 diabetes, beyond that provided by clinical risk factors.
Methods: NT-proBNP was measured in 1993 samples from the Hong Kong Diabetes Biobank, a multicentre prospective diabetes cohort and biobank. A cut-off of ≥125 pg/ml was used to define elevated NT-proBNP. Associations between elevated NT-proBNP and incident cardiovascular and renal endpoints were examined using Cox regression, adjusted for sex, age and duration of diabetes, as well as other covariates. Prognostic and incremental predictive values of NT-proBNP in diabetes cardiorenal complications, compared with those of the Joint Asia Diabetes Evaluation risk equations for CHD, CHF and kidney failure, were evaluated using the concordance index (C index), net reclassification improvement index, integrated discrimination improvement index and relative integrated discrimination improvement index.
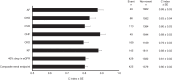
Results: A total of 24.7% of participants had elevated NT-proBNP. Participants with elevated NT-proBNP at baseline had a more adverse cardiometabolic profile, with 2-4-fold higher frequency of complications at baseline. Adjusting for age at baseline, sex and duration of diabetes, elevated NT-proBNP was associated with incident atrial fibrillation (HR 4.64 [95% CI 2.44, 8.85]), CHD (HR 4.21 [2.46, 7.21]), CVD (HR 3.32 [2.20, 5.01]) and CHF (HR 4.18 [2.18, 8.03]; all p<0.001). All these associations remained significant after further adjustment for additional covariates. Elevated NT-proBNP had good discriminative ability for various cardiorenal endpoints, with C index of 0.83 (95% CI 0.76, 0.90) for CHD, 0.88 (0.81, 0.94) for atrial fibrillation, 0.89 (0.83, 0.95) for CHF, 0.81 (0.77, 0.84) for 40% drop in eGFR and 0.88 (0.84, 0.92) for kidney failure. Models incorporating NT-proBNP had improved prediction compared with established clinical risk models. Sensitivity analyses including alternative cut-off of NT-proBNP, as well as use of other risk engines of CHD, yielded similar results.
Conclusions/interpretation: NT-proBNP demonstrated a promising ability to serve as a prognostic marker for a variety of cardiorenal complications in type 2 diabetes. Considering NT-proBNP in clinical assessments could potentially help identify high-risk individuals who may benefit from more intensive therapies.
Keywords: Biomarkers; Cardiovascular; Complications; Epidemiology; Heart failure; Precision medicine.
© 2024. The Author(s).
Conflict of interest statement
Acknowledgements: The authors thank all the participants and investigators of the HKDB for their contributions, and the medical and nursing staff of the diabetes centres involved for their professional input and support. Some of the data were presented as an abstract at the ADA's 83rd Scientific Sessions in 2023. Data availability: The datasets used during the current study are available from the corresponding author (RCWM) upon reasonable request. Funding: The measurement of NT-proBNP in the Hong Kong Diabetes Biobank was supported by an investigator-initiated grant from Roche Diagnostics (Hong Kong) Limited (to RCWM). Additional support for the Hong Kong Diabetes Biobank and this project came from the Research Grants Council of the Hong Kong Special Administrative Region (CU R4012-18), the Research Grants Council Theme-based Research Scheme (T12-402/13N), the Focused Innovation Scheme, the University Research Grants Matching Scheme and a Croucher Foundation Senior Medical Research Fellowship. The funding sources did not have any role in the design, interpretation of the study results, or the decision to publish the results. Authors' relationships and activities: JCNC received consultancy fees or speaker honoraria from Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Celltrion, Eli Lilly, Hua Medicine, Lee Power Pharmaceuticals, Merck, MSD, Pfizer, Roche, Sanofi, Servier, Viatris Pharmaceutical and Zuellig Pharma. JCNC is the Chief Executive Officer (pro bono) of the Asian Diabetes Foundation. APSK received consultancy fees, speaker honoraria and/or research grants from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, Kyowa Kirin, Merck Serono, Merck Sharp & Dohme, Nestle, Novo Nordisk, Pfizer, Sanofi and Zuellig Pharma. EC received speaker fees from Sanofi and Novartis, and institutional research funding from Sanofi, Medtronic Diabetes and Powder Pharmaceuticals, Inc. RCWM received research funding from AstraZeneca, Bayer, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Roche Diagnostics and Tricida, Inc. for carrying out clinical trials or studies, and from AstraZeneca, Bayer, Boehringer Ingelheim and Merck for speaker honoraria or consultancy in advisory boards. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. RCWM is a member of the Editorial Board of Diabetologia. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: Funding acquisition and overall project support were provided by RCWM. Conception and design of the work was provided by RCWM and CKPL. RCWM, YH, ESHL, RO, ACWN, AOYL, WYS, CKPL and JCNC were responsible for data collection and project logistics. RCWM, CHTT, YH, ESHL, JNML, EC, APSK, CH, EGF, ACWN and CKPL conducted the data analysis or data interpretation. RCWM and CHTT drafted the manuscript. RCWM, CHTT, YH, ESHL, RO, JNML, EC, APSK, CH, ACWN, EGF, AOYL, WYS, CKPL and JCNC provided critical revisions and contributed to the writing of the manuscript. All authors approved the final version of the article to be published. RCWM is responsible for the integrity of the work as a whole.
Figures
References
-
- de Boer IH, Khunti K, Sadusky T et al (2022) Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 102(5):974–989. 10.1016/j.kint.2022.08.012 - PubMed
-
- Dennis JM, Young KG, McGovern AP et al (2022) Development of a treatment selection algorithm for SGLT2 and DPP-4 inhibitor therapies in people with type 2 diabetes: a retrospective cohort study. Lancet Digit Health 4(12):e873–e883. 10.1016/S2589-7500(22)00174-1 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Croucher Foundation Senior Medical Research Fellow/Croucher Foundation
- Research Impact Fund (CU R4012-18)/Research Grants Council, University Grants Committee
- Theme-based Research Scheme (T12-402/13N)/Research Grants Council, University Grants Committee
- University Research Grants Matching Scheme/Research Grants Council, University Grants Committee
- Focused Innovation Scheme/Chinese University of Hong Kong
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

